Medicare Plus Blue PPO Assure & PDP Option B. Prior Authorization/Step Therapy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Quarterly Review
Tropical Gastroenterology 2008.29;4:187–193 Quarterly Have hematopoietic growth factors made an Review impact on the management of liver disease? Pankaj Tyagi and Kaushal Madan ABSTRACT Department of Gastroenterology, It is clear that the major indication for the use of hematopoietic growth factors in hepatology GB Pant Hospital & Department of is to counteract the adverse effects of interferons (neutropenia and thrombocytopenia) and Medical Hepatology, ribavirin (hemolytic anaemia) during the treatment of hepatitis C infection. This is important Institute of Liver and Biliary Sciences, because the probability of SVR depends on proper adherence to therapy (at least 80% of the New Delhi requisite dose maintained for at least 80% of the requisite duration) and proper adherence can only be achieved if the side effects are reduced to a minimum. Even though the studies Correspondence: Dr. Kaushal Madan have demonstrated beyond doubt that the use of hematopoietic growth factors does indeed Email: [email protected] reduce the incidence and severity of these adverse effects and helps the patients to complete the course of therapy, the data on improvement of SVR is still limited. There is only one study of darbepoetin and filgrastim showing the beneficial effect on SVR. Even among the hematological side effects, possibly the only significant effect which limits the use of optimal HCV therapy is the hemolytic anaemia induced by ribavirin. The other two main side effects, i.e. neutropenia and thrombocytopenia are not clinically problematic. The use of such growth factors would be particularly effective if patients who have advanced liver disease or cirrhosis are able to receive adequate anti-viral therapy as has been demonstrated in the study of eltrombopag among HCV cirrhotics. -

September 11, 2018 DUR Minutes
Maine Department of Health and Human Services PAUL R. LEPAGE MaineCare Services BETHANY L. HAMM GOVERNOR Pharmacy Unit ACTING COMMISSIONER 11 State House Station Augusta, Maine 04333-0011 TO: Maine Drug Utilization Review Board DATE: 9/14/2018 RE: Maine DUR Board Meeting minutes from September 11, 2018 ATTENDANCE PRESENT ABSENT EXCUSED Linda Glass, MD X Lisa Wendler, Pharm. D., Clinical Pharmacy Specialist, X Maine Medical CTR Mike Antoniello, MD X Kathleen Polonchek, MD X Kenneth McCall, PharmD X Steve Diaz, MD X Erin Ackley, PharmD. X Corinn Martineau, PharmD. X Non –Voting Mike Ouellette, R.Ph., Change Healthcare X Jeffery Barkin, MD, Change Healthcare X Christopher Pezzullo, State Health Officer DHHS, DO X Jill Kingsbury, MaineCare Pharmacy Director X Guests of the Board: Ed Bosshart, PharmD, Jeff Caulfield, Lead Epidemiologist for Viral Infections from CDC: Discussed HCV treatment. CALL TO ORDER: 5:30PM Jill Kingsbury called the meeting to order at 5:30 PM. PUBLIC COMMENTS Robert Mead from Pfizer: Highlighted the attributes of Retacrit. Jane Guo from Otsuka: Highlighted the attributes of Jynarque. OLD BUSINESS DUR MINUTES The June DUR meeting minutes were accepted as written. MAINECARE UPDATE No update at this time. NEW BUSINESS INTRODUCTION: USE OF CHRONIC TRIPTANS The use of triptans has become standard of care for the treatment of acute migraine headaches, given their effectiveness, safety and tolerability. However, like many medications used to treat migraine, overuse renders them less effective. Additionally, rebound headaches from triptan overuse is common. For patients who experience frequent headaches, or whose headaches are long lasting or chronic, use of headache prophylactic medications are recommended by several medical associations, including the American Headache Society and the American Academy of Neurology. -

Managing Adverse Effects and Complications in Completing Treatment for Hepatitis C Virus Infection
HCV Treatment Complications Volume 20 Issue 4 October/November 2012 Perspective Managing Adverse Effects and Complications in Completing Treatment for Hepatitis C Virus Infection The addition of direct-acting antivirals (DAAs) to hepatitis C virus (HCV) Psychiatric Complications treatment regimens has made treatment more effective and patient Depression is the most common psy- management more complex. Shepherding patients through a full course of chiatric complication encountered in HCV therapy requires motivation and involvement on the part of the patient HCV patients, with mild to moderate and the physician. Indeed, physician inexperience and lack of confidence in depression found in as much as 80% of guiding patients through the challenges of treatment appears to be a primary patients. Bipolar disorder and schizo- reason for early discontinuation of therapy. Among the many complications phrenia are also not infrequently en- of HCV treatment that must be managed efficiently and effectively are countered. depression and other psychiatric disorders; hematologic abnormalities There is little evidence to support including DAA- and ribavirin-associated anemia and peginterferon alfa- a benefit of preemptive antidepres- associated neutropenia and thrombocytopenia; rash and drug eruptions, sant therapy in all patients undergo- including telaprevir-associated rash; and weight loss. Practical considerations ing HCV treatment, though a recent in management of these common complications are offered. This article randomized trial of HCV patients -

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

TEXAS MEDICAID Clinical Edit Prior Authorization Epoetin Alfa (PROCRIT)
TEXAS MEDICAID Clinical Edit Prior Authorization epoetin alfa (PROCRIT) STEP 1: CLEARLY PRINT AND COMPLETE TO EXPEDITE PROCESSING Date: Prescriber First & Last Name: Patient First & Last Name: Prescriber NPI: Patient Address: Prescriber Address: Patient ID: Prescriber Phone: Patient Date of Birth: Prescriber Fax: STEP 2: MEDICATION INFORMATION Medication Requested (Name): Quantity Requested: Dose Requested: Dosing Instructions: Patient’s Primary Diagnosis: ____________________________________ ICD 10 Code: __________ Please indicate ONE (1) of the following: OR STAR / STAR KIDS client (Go to Step 3 - PDL PA Criteria Applies) OR CHIP / PERINATE client (Go to Step 4) STEP 3: PDL PRIOR AUTHORIZATION CRITERIA FOR NON-PREFERRED PRODUCT 1. Has the client failed a 30-day treatment trial with at least 1 preferred agent in the last 180 days? Yes (Go to Step 4 Question 1) No (Go to #2) 2. Is there a documented allergy or contraindication to preferred agents in this class? Yes (Go to Step 4 Question 1) No (Go to #3) 3. Is the drug necessary for treatment of stage-4 advanced metastatic cancer and associated conditions? Yes (Go to Step 4 Question 1) No (Deny) Rev. 11/18/2020 Page 1 of 3 Version 1.5 STEP 4: CLINICAL PRIOR AUTHORIZATION CRITERIA 1. Does the client have a diagnosis of chronic renal failure in the last 730 days? Yes (Go to #7) No (Go to #2) 2. Does the client have a diagnosis of cancer in the last 730 days? Yes (Go to #3) No (Go to #5) 3. Does the client have a history of an antineoplastic agent in the last 30 days? Examples of antineoplastic -

UWHC Guidelines for the Use of Darbepoetin and Epoetin
Use of Darbepoetin and Epoetin in Non-Nephrology Patients – Adult/Pediatric – Inpatient/Ambulatory Clinical Practice Guideline Table of Contents Executive Summary ......................................................................................... 3 Scope ............................................................................................................. 7 Methodology ................................................................................................... 7 Definitions (optional): ...................................................................................... 8 Introduction.................................................................................................... 8 Recommendations........................................................................................... 9 UW Health Implementation............................................................................ 19 References ................................................................................................... 19 Note: Active Table of Contents Click to follow link CPG Contact for Changes: Name: Philip Trapskin, PharmD, BCPS, Manager, DPP Phone Number: 608-263-1328 Email address: [email protected] CPG Contact for Content: Name: Jason Bergsbaken, PharmD Phone Number: 608-265-0341 Email address: [email protected] Copyright © 2015 University of Wisconsin Hospitals and Clinics Authority Contact: [email protected] Vermeulen, [email protected] Last Revised: 07/2015 Guideline Authors: Jason Bergsbaken, PharmD Coordinating -

Investor Presentation
Participants Company overview Pharmaceuticals Oncology Financial review Conclusion Appendix References Q1 2021 Results Investor presentation 1 Investor Relations │ Q1 2021 Results Participants Company overview Pharmaceuticals Oncology Financial review Conclusion Appendix References Disclaimer This presentation contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995, that can generally be identified by words such as “potential,” “expected,” “will,” “planned,” “pipeline,” “outlook,” or similar expressions, or by express or implied discussions regarding potential new products, potential new indications for existing products, potential product launches, or regarding potential future revenues from any such products; or regarding the impact of the COVID-19 pandemic on certain therapeutic areas including dermatology, ophthalmology, our breast cancer portfolio, some newly launched brands and the Sandoz retail and anti-infectives business, and on drug development operations; or regarding potential future, pending or announced transactions; regarding potential future sales or earnings of the Group or any of its divisions; or by discussions of strategy, plans, expectations or intentions; or regarding the Group’s liquidity or cash flow positions and its ability to meet its ongoing financial obligations and operational needs; or regarding our collaboration with Molecular Partners to develop, manufacture and commercialize potential medicines for the prevention and treatment of COVID- 19 and our joining of the industry-wide efforts to meet global demand for COVID-19 vaccines and therapeutics by leveraging our manufacturing capacity and capabilities to support the production of the Pfizer-BioNTech vaccine and to manufacture the mRNA and bulk drug product for the vaccine candidate CVnCoV from CureVac. -

Dr. Winegarden Presentation
Empowering Market Competition through Biosimilars Presentation to National Council of Insurance Legislators (NCOIL) 2019 Summer Meeting Newport Beach, California July 12, 2019 Total Savings (in millions) 25% 50% 75% Originator Total Annual Savings for Current, 25%, Drug Class Current Biosimilar Biosimilar Biosimilar Biologic 50%, and 75% Biosimilar Share Share Share Share Scenarios Compared to All-Originator Infliximab Remicade $79.4 $318.2 $636.5 $954.7 Biologic Baseline Pegfilgrastim Neulasta $21.8 $121.9 $243.8 $365.7 Filgrastim Neupogen $152.1 $152.1 $152.1 $206.8 Epoetin Alfa Epogen & Procrit $0.5 $8.4 $16.9 $25.3 Bevacizumab Avastin $0.0 $199.2 $398.5 $597.7 Trastuzumab Herceptin $0.0 $208.0 $415.9 $623.9 • Biosimilars price discounts expected 30% - Rituxumab Rituxan $0.0 $280.6 $561.2 $841.8 40% off originator biologic Etanercept Enbrel $0.0 $324.0 $648.0 $972.1 • Forthcoming study estimates possible Adalimunab Humira $0.0 $861.1 $1,722.1 $2,583.2 health care savings from wider adoption of biosimilars. GRAND TOTAL $253.8 $2,473.6 $4,795.0 $7,171.2 • Estimates are based on the average sales price (ASP) data that are effective from April 2019 through June 2019 and rolling 12-month volume data through February 2019. Biosimilars have no “clinically meaningful difference” in safety, purity, • Methodology: compare potential savings to hypothetical all-originator biologic scenario and effectiveness relative to its reference originator biologic. Just like • Over 10 years, potential savings of $24.7 generic medicines, the benefits of biosimilars are the substantial price billion, $48.0 billion, and $71.7 billion respectively. -
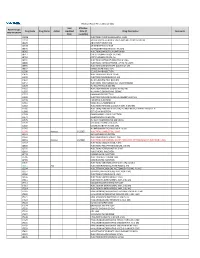
2021 Prior Authorization List Part B Appendix a (PDF)
Medicare Part B PA List Effective 2021 Last Effective Part B Drugs: Drug Code Drug Name Action Updated Date (if Drug Description Comments STEP THERAPY Date available) C9050 INJECTION, EMAPALUMAB-LZSG, 1 MG C9122 MOMETASONE FUROATE SINUS IMPLANT 10 MCG SINUVA J0129 ABATACEPT INJECTION J0178 AFLIBERCEPT INJECTION J0570 BUPRENORPHINE IMPLANT 74.2MG J0585 INJECTION,ONABOTULINUMTOXINA J0717 CERTOLIZUMAB PEGOL INJ 1MG J0718 CERTOLIZUMAB PEGOL INJ J0791 INJECTION CRIZANLIZUMAB-TMCA 5 MG J0800 INJECTION, CORTICOTROPIN, UP TO 40 UNITS J0896 INJECTION LUSPATERCEPT-AAMT 0.25 MG J0897 DENOSUMAB INJECTION J1300 ECULIZUMAB INJECTION J1428 INJECTION ETEPLIRSEN 10 MG J1429 INJECTION GOLODIRSEN 10 MG J1442 INJ FILGRASTIM EXCL BIOSIMIL J1447 INJECTION, TBO-FILGRASTIM, 1 MICROGRAM J1459 INJ IVIG PRIVIGEN 500 MG J1555 INJECTION IMMUNE GLOBULIN 100 MG J1556 INJ, IMM GLOB BIVIGAM, 500MG J1557 GAMMAPLEX INJECTION J1558 INJECTION IMMUNE GLOBULIN XEMBIFY 100 MG J1559 HIZENTRA INJECTION J1561 GAMUNEX-C/GAMMAKED J1562 INJECTION; IMMUNE GLOBULIN 10%, 5 GRAMS J1566 INJECTION, IMMUNE GLOBULIN, INTRAVENOUS, LYOPHILIZED (E.G. P J1568 OCTAGAM INJECTION J1569 GAMMAGARD LIQUID INJECTION J1572 FLEBOGAMMA INJECTION J1575 INJ IG/HYALURONIDASE 100 MG IG J1599 IVIG NON-LYOPHILIZED, NOS J1602 GOLIMUMAB FOR IV USE 1MG J1745 INJ INFLIXIMAB EXCL BIOSIMILR 10 MG J1930 Remove 1/1/2021 INJECTION, LANREOTIDE, 1 MG J2323 NATALIZUMAB INJECTION J2350 INJECTION OCRELIZUMAB 1 MG J2353 Remove 1/1/2021 INJECTION, OCTREOTIDE, DEPOT FORM FOR INTRAMUSCULAR INJECTION, 1 MG J2357 INJECTION, OMALIZUMAB, -

Summary of Appeals & Independent Review Organization
All Other Appeals All other appeals are for drugs not in an inpatient hospital setting that Molina was not able to approve. Sometimes, the clinical information sent to us for these drugs do not meet medical necessity on initial review. When drug preauthorization requests are denied, a member or provider has the right to appeal. Appeals allow time to provide more clinical information. With complete clinical information, we can usually approve the drug. These are considered an appeal overturn. When the denial decision is not overturned, it is considered upheld. Service Code/Drug Name Service Code Description Number of Appeals Number of Appeals Total Appeals Upheld Overturned A9274 EXTERNAL AMB INSULIN DEL SYSTEM DISPOSABLE EA 0 1 1 Abatacept 3 1 4 Abemaciclib 1 0 1 Acalabrutinib 0 1 1 Acne Combination - Two Ingredient 1 0 1 Acyclovir 0 1 1 Adalimumab 7 14 21 Adrenergic Combination - Two Ingredient 1 0 1 Aflibercept 0 2 2 Agalsidase 1 0 1 Alfuzosin 1 0 1 Amantadine 1 0 1 Ambrisentan 0 1 1 Amphetamine 0 1 1 Amphetamine Mixtures - Two Ingredient 1 8 9 Apixaban 7 21 28 Apremilast 12 13 25 Aprepitant 0 1 1 Aripiprazole 5 9 14 ARNI-Angiotensin II Recept Antag Comb - Two Ingredient 6 9 15 Asenapine 0 1 1 Atomoxetine 1 3 4 Atorvastatin 0 1 1 Atovaquone 1 0 1 Axitinib 0 1 1 Azathioprine 0 1 1 Azilsartan 1 0 1 Azithromycin 1 0 1 Baclofen 0 1 1 Baricitinib 1 1 2 Belimumab 0 1 1 Benralizumab 1 0 1 Beta-blockers - Ophthalmic Combination - Two Ingredient 0 2 2 Bimatoprost 0 1 1 Botulinum Toxin 1 4 5 Buprenorphine 4 3 7 Calcifediol 1 0 1 Calcipotriene -

And Intermediate-1-Risk MDS
POST-ASH Issue 4, 2016 Initial Results from Phase II Trials of Eltrombopag or Luspatercept for Myelosuppression in Low- and Intermediate-1-Risk MDS For more visit ResearchToPractice.com/5MJCASH2016 CME INFORMATION OVERVIEW OF ACTIVITY Each year, thousands of clinicians, basic scientists and other industry professionals sojourn to major international oncology conferences, like the American Society of Hematology (ASH) annual meeting, to hone their skills, network with colleagues and learn about recent advances altering state-of-the-art management in hematologic oncology. These events have become global stages where exciting science, cutting-edge concepts and practice-changing data emerge on a truly grand scale. This massive outpouring of information has enormous benefits for the hematologic oncology community, but the truth is it also creates a major challenge for practicing oncologists and hematologists. Although original data are consistently being presented and published, the flood of information unveiled during a major academic conference is unmatched and leaves in its wake an enormous volume of new knowledge that practicing oncologists must try to sift through, evaluate and consider applying. Unfortunately and quite commonly, time constraints and an inability to access these data sets leave many oncologists struggling to ensure that they’re aware of crucial practice-altering findings. This creates an almost insurmountable obstacle for clinicians in community practice because they are not only confronted almost overnight with thousands -

Presentation Title
The COMMANDS trial: a phase 3 study of the efficacy and safety of luspatercept versus epoetin alfa for the treatment of anemia due to Revised International Prognostic Scoring System Very Low-, Low-, or Intermediate-risk myelodysplastic syndromes in erythropoiesis stimulating agent-naive patients who require red blood cell transfusions Matteo Della Porta,1,2 Uwe Platzbecker,3 Valeria Santini,4 Guillermo Garcia-Manero,5 Rami S. Komrokji,6 Rodrigo Ito,7 Pierre Fenaux8 1Cancer Center IRCCS Humanitas Research Hospital, Milan, Italy; 2Department of Biomedical Sciences, Humanitas University, Milan, Italy; 3Medical Clinic and Policlinic 1, Hematology and Cellular Therapy, University Hospital Leipzig, Leipzig, Germany; 4Azienda Ospedaliero-Universitaria Careggi, University of Florence, Florence, Italy; 5Department of Leukemia, University of Texas MD Anderson Cancer Center, Houston, TX; 6Moffitt Cancer Center, Tampa, FL; 7Bristol Myers Squibb, Princeton, NJ; 8Service d'Hématologie Séniors, Hôpital Saint-Louis, Université Paris 7, Paris, France Presentation 2198 Presenting author disclosures M.D.P.: no conflicts of interest to disclose. 2 Introduction and objectives Introduction • Studies of epoetin alfa and darbepoetin alfa have demonstrated efficacy among patients with LR-MDS, but the patient population in which a clinically significant effect is observed may be limited1,2 • Luspatercept, a first-in-class erythroid maturation agent with a mechanism of action distinct from ESAs,3 is approved by the US FDA for the treatment of anemia failing an