Self-Administered Specialty Drug List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
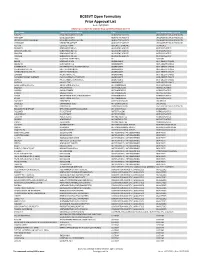
BCBSVT Open Formulary Prior Approval List
BCBSVT Open Formulary Prior Approval List As of: 10/27/2020 Helpful Tip: To search for a specific drug, use the find feature (Ctrl + F) Trade Name Chemical/Biological Name Class Prior Authorization Program FUSILEV LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS KHAPZORY LEVOLEUCOVORIN ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS LEVOLEUCOVORIN CALCIUM LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS VISTOGARD URIDINE TRIACETATE ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS ACTHAR CORTICOTROPIN ADRENAL HORMONES HORMONES BELRAPZO BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDAMUSTINE HCL BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDEKA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS TREANDA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS DAW (DISPENSE AS WRITTEN) ALL CUSTOM BELVIQ LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS BELVIQ XR LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS CONTRAVE ER NALTREXONE HCL/BUPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL ER DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS LOMAIRA PHENTERMINE HCL ANOREXIANTS ANTI‐OBESITY DRUGS PHENDIMETRAZINE TARTRATE PHENDIMETRAZINE TARTRATE ANOREXIANTS ANTI‐OBESITY DRUGS QSYMIA PHENTERMINE/TOPIRAMATE ANOREXIANTS ANTI‐OBESITY DRUGS SAXENDA LIRAGLUTIDE ANOREXIANTS ANTI‐OBESITY DRUGS ABIRATERONE ACETATE ABIRATERONE ACETATE ANTIANDROGENS ANTINEOPLASTICS ERLEADA APALUTAMIDE ANTIANDROGENS ANTINEOPLASTICS NUBEQA DAROLUTAMIDE ANTIANDROGENS -

September 11, 2018 DUR Minutes
Maine Department of Health and Human Services PAUL R. LEPAGE MaineCare Services BETHANY L. HAMM GOVERNOR Pharmacy Unit ACTING COMMISSIONER 11 State House Station Augusta, Maine 04333-0011 TO: Maine Drug Utilization Review Board DATE: 9/14/2018 RE: Maine DUR Board Meeting minutes from September 11, 2018 ATTENDANCE PRESENT ABSENT EXCUSED Linda Glass, MD X Lisa Wendler, Pharm. D., Clinical Pharmacy Specialist, X Maine Medical CTR Mike Antoniello, MD X Kathleen Polonchek, MD X Kenneth McCall, PharmD X Steve Diaz, MD X Erin Ackley, PharmD. X Corinn Martineau, PharmD. X Non –Voting Mike Ouellette, R.Ph., Change Healthcare X Jeffery Barkin, MD, Change Healthcare X Christopher Pezzullo, State Health Officer DHHS, DO X Jill Kingsbury, MaineCare Pharmacy Director X Guests of the Board: Ed Bosshart, PharmD, Jeff Caulfield, Lead Epidemiologist for Viral Infections from CDC: Discussed HCV treatment. CALL TO ORDER: 5:30PM Jill Kingsbury called the meeting to order at 5:30 PM. PUBLIC COMMENTS Robert Mead from Pfizer: Highlighted the attributes of Retacrit. Jane Guo from Otsuka: Highlighted the attributes of Jynarque. OLD BUSINESS DUR MINUTES The June DUR meeting minutes were accepted as written. MAINECARE UPDATE No update at this time. NEW BUSINESS INTRODUCTION: USE OF CHRONIC TRIPTANS The use of triptans has become standard of care for the treatment of acute migraine headaches, given their effectiveness, safety and tolerability. However, like many medications used to treat migraine, overuse renders them less effective. Additionally, rebound headaches from triptan overuse is common. For patients who experience frequent headaches, or whose headaches are long lasting or chronic, use of headache prophylactic medications are recommended by several medical associations, including the American Headache Society and the American Academy of Neurology. -

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

TEXAS MEDICAID Clinical Edit Prior Authorization Epoetin Alfa (PROCRIT)
TEXAS MEDICAID Clinical Edit Prior Authorization epoetin alfa (PROCRIT) STEP 1: CLEARLY PRINT AND COMPLETE TO EXPEDITE PROCESSING Date: Prescriber First & Last Name: Patient First & Last Name: Prescriber NPI: Patient Address: Prescriber Address: Patient ID: Prescriber Phone: Patient Date of Birth: Prescriber Fax: STEP 2: MEDICATION INFORMATION Medication Requested (Name): Quantity Requested: Dose Requested: Dosing Instructions: Patient’s Primary Diagnosis: ____________________________________ ICD 10 Code: __________ Please indicate ONE (1) of the following: OR STAR / STAR KIDS client (Go to Step 3 - PDL PA Criteria Applies) OR CHIP / PERINATE client (Go to Step 4) STEP 3: PDL PRIOR AUTHORIZATION CRITERIA FOR NON-PREFERRED PRODUCT 1. Has the client failed a 30-day treatment trial with at least 1 preferred agent in the last 180 days? Yes (Go to Step 4 Question 1) No (Go to #2) 2. Is there a documented allergy or contraindication to preferred agents in this class? Yes (Go to Step 4 Question 1) No (Go to #3) 3. Is the drug necessary for treatment of stage-4 advanced metastatic cancer and associated conditions? Yes (Go to Step 4 Question 1) No (Deny) Rev. 11/18/2020 Page 1 of 3 Version 1.5 STEP 4: CLINICAL PRIOR AUTHORIZATION CRITERIA 1. Does the client have a diagnosis of chronic renal failure in the last 730 days? Yes (Go to #7) No (Go to #2) 2. Does the client have a diagnosis of cancer in the last 730 days? Yes (Go to #3) No (Go to #5) 3. Does the client have a history of an antineoplastic agent in the last 30 days? Examples of antineoplastic -

Freedom of Information Act 2000
FREEDOM OF INFORMATION ACT 2000 THE ROYAL CORNWALL HOSPITALS NHS TRUST RESPONSE TO INFORMATION REQUEST Date Request Received: 15th November 2019 FOI Ref: 8454 Requested Information Question for your pharmacy and/or procurement team regarding the number of medicines and/or nursing services provided to NHS patients by an Independent Homecare Provider 1) In your organisation, which named individuals have the overall responsibility for any homecare provision for your patients? 2) Do you currently have in post an operational lead for homecare services in your organisation – If so, what is their name/role? 3) What are your organisations minimum requirements for accepting a homecare provider? 4) If you have an outsourced outpatient pharmacy, are they able to provide nurse services / training for patients on how to self-inject for medicines administered by sub-cutaneous injection as part of their contract? Can you please advise of total numbers of NHS patients who; 5) Received a homecare delivery service of drug and/or nurse service at dates Jan 2018 / Jan 2019 / October 2019 – please provide these numbers by; a) Drug name b) Therapy / clinical area c) Name of the homecare provider who provided/provides this service d) If possible please identify if these services are NHS funded or pharmaceutical / manufacturer funded services. Response 1) The Chief Pharmacist at the Royal Cornwall Hospitals Trust has overall responsibility for Medicines Homecare services. 2) The Royal Cornwall Hospitals Trust Chief Pharmacy Technician is the Operational Lead -

Neuromyelitis Optica Spectrum Disorder
© Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 | Fax 503-947-2596 Drug Class Review with New Drug Evaluation: Biologics for Autoimmune Disorders-Neuromyelitis Optica Spectrum Disorder Date of Review: April 2021 Date of Last Review: n/a Dates of Literature Search: 1/1/1996 – 1/20/2021 Generic Name: Brand Name (Manufacturer): Eculizumab Soliris® (Alexion Pharmaceuticals) Inebilizumab-cdon Uplizna™ (Viela Bio) Satralizumab-mwge Enspryng™ (Genentech/Roche) Dossiers Received: Yes Current Status of PDL Class: See Appendix 1. Purpose for Class Update: To define place in therapy for 3 immunosuppressive agents, eculizumab, inebilizumab-cdon, and satralizumab-mwge, recently approved by the Food and Drug Administration (FDA) for the treatment adults with neuromyelitis optica spectrum disorder (NMOSD). Research Questions: 1. What is the effectiveness of eculizumab, inebilizumab, and satralizumab in reducing time to relapse in adult patients with NMOSD who are anti-aquaporin-4 (AQP4) antibody positive? 2. What are the harms of eculizumab, inebilizumab-cdon and satralizumab in adults with NMOSD? 3. Is there comparative evidence that eculizumab, inebilizumab, and satralizumab differ in efficacy or harms for management of NMOSD? 4. Are there certain sub-populations (based on age, gender, ethnicity, comorbidities, disease duration or severity) in which eculizumab, inebilizumab, or satralizumab may be beneficial -

FLT3 Inhibitors in Acute Myeloid Leukemia Mei Wu1, Chuntuan Li2 and Xiongpeng Zhu2*
Wu et al. Journal of Hematology & Oncology (2018) 11:133 https://doi.org/10.1186/s13045-018-0675-4 REVIEW Open Access FLT3 inhibitors in acute myeloid leukemia Mei Wu1, Chuntuan Li2 and Xiongpeng Zhu2* Abstract FLT3 mutations are one of the most common findings in acute myeloid leukemia (AML). FLT3 inhibitors have been in active clinical development. Midostaurin as the first-in-class FLT3 inhibitor has been approved for treatment of patients with FLT3-mutated AML. In this review, we summarized the preclinical and clinical studies on new FLT3 inhibitors, including sorafenib, lestaurtinib, sunitinib, tandutinib, quizartinib, midostaurin, gilteritinib, crenolanib, cabozantinib, Sel24-B489, G-749, AMG 925, TTT-3002, and FF-10101. New generation FLT3 inhibitors and combination therapies may overcome resistance to first-generation agents. Keywords: FMS-like tyrosine kinase 3 inhibitors, Acute myeloid leukemia, Midostaurin, FLT3 Introduction RAS, MEK, and PI3K/AKT pathways [10], and ultim- Acute myeloid leukemia (AML) remains a highly resist- ately causes suppression of apoptosis and differentiation ant disease to conventional chemotherapy, with a me- of leukemic cells, including dysregulation of leukemic dian survival of only 4 months for relapsed and/or cell proliferation [11]. refractory disease [1]. Molecular profiling by PCR and Multiple FLT3 inhibitors are in clinical trials for treat- next-generation sequencing has revealed a variety of re- ing patients with FLT3/ITD-mutated AML. In this re- current gene mutations [2–4]. New agents are rapidly view, we summarized the preclinical and clinical studies emerging as targeted therapy for high-risk AML [5, 6]. on new FLT3 inhibitors, including sorafenib, lestaurtinib, In 1996, FMS-like tyrosine kinase 3/internal tandem du- sunitinib, tandutinib, quizartinib, midostaurin, gilteriti- plication (FLT3/ITD) was first recognized as a frequently nib, crenolanib, cabozantinib, Sel24-B489, G-749, AMG mutated gene in AML [7]. -

Neuromyelitis Optica News
Neuromyelitis Optica News Gloria von Geldern, MD Assistant Professor of Neurology University of Washington Multiple Sclerosis Center Multiple Sclerosis Regional Summit June 13, 2020 Disclosures ▪ No conflict of interest ▪ Off-label treatments will be mentioned Current research funding Patient-Centered Outcomes Research Institute (PCORI) What is New in NMO? Natalia Rost, MD Chair scientific committee AAN Neuromyelitis Optica (NMO) Neuromyelitis Optica Spectrum Disease (NMOSD) ▪ Inflammatory disorder of the central nervous system ▪ Predominantly involving optic nerves and spinal cord ▪ Severe demyelination and axonal damage ▪ Rare disease: 0.5-10/100,000, ~8,000 patients in the US ▪ Black > Asian > White ▪ Female > Male Flanagan et al., 2016 Bukhari et al. 2017 Hor et al. 2018 (Guthy-Jackson Charitable Foundation) Multiple Sclerosis vs NMO Pittock and Lucchinetti 2016 History 1894: Clinical case descriptions 2004: AQP4 antibody identified by Devic and Gault by Lennon, Weinshenker et al. Eugene Devic (1858–1930) Lancet2004; 364: 2106–12; Journal of Neuroinflammation 2013, 10:8 Diagnostic Criteria Wingerchuk et al. 2015 Myelin Oligodendrocyte (MOG) Antibody ▪ More common in men and whites ▪ Bilateral optic neuritis, caudal myelitis ▪ Better recovery compared to AQP4-Ab pos NMO ▪ MRI brain without NMO findings ▪ Assay now commercially available at Mayo Sato et al. 2014; van Pelt et al. 2016 Auto-Antibody to Aquaporin 4 Water channel protein in ▪ Astrocytic foot processes at the blood-brain barrier ▪ Spinal cord grey matter ▪ Periaqueductal and periventricular regions ▪ Mueller cells in the retina Amiry-Moghaddam and Otterson 2003 Bystander Oligodendrocyte Injury Tradtrantip et al 2017 Pathology Extensive demyelination Perivascular & parenchymal Complement activation Axonal injury, necrosis eosinophils & granulocytes rosette and rim pattern Jacob et al. -

Biosimilar Epoetins and Other ``Follow-On
Biosimilar epoetins and other “follow-on” biologics: Update on the European experiences Wolfgang Jelkmann To cite this version: Wolfgang Jelkmann. Biosimilar epoetins and other “follow-on” biologics: Update on the European experiences. American Journal of Hematology, Wiley, 2010, 85 (10), pp.771. 10.1002/ajh.21805. hal-00552331 HAL Id: hal-00552331 https://hal.archives-ouvertes.fr/hal-00552331 Submitted on 6 Jan 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. American Journal of Hematology Biosimilar epoetins and other “follow-on” biologics: Update on the European experiences For Peer Review Journal: American Journal of Hematology Manuscript ID: AJH-10-0229.R1 Wiley - Manuscript type: Critical Review Date Submitted by the 10-Jun-2010 Author: Complete List of Authors: Jelkmann, Wolfgang; University, Physiology Anemias, Erythropoietin, Hematology- medical, Neutropenia, Keywords: Pharmacology John Wiley & Sons Page 1 of 30 American Journal of Hematology 1 2 3 Table II. Benefits and problems related to the use of biosimilars 4 5 ________________________________________________________________ 6 Benefits Problems 7 ______________________________________________________________________ 8 9 10 Lower pricing than originator medicines Lack of long-term experience 11 (efficacy, safety, immunogenicity?) 12 13 Pressure on innovator companies Product-specific administration routes 14 15 to reduce prices of originator medicines (s.c. -

Federal Register Notice 5-1-2020 Pdf Icon[PDF – 358
Federal Register / Vol. 85, No. 85 / Friday, May 1, 2020 / Notices 25439 confidential by the respondent (5 U.S.C. schedules. Other than examination DEPARTMENT OF HEALTH AND 552(b)(4)). reports, it provides the only financial HUMAN SERVICES Current actions: The Board has data available for these corporations. temporarily revised the instructions to The Federal Reserve is solely Centers for Disease Control and the FR Y–9C report to accurately reflect responsible for authorizing, supervising, Prevention the revised definition of ‘‘savings and assigning ratings to Edges. The [CDC–2020–0046; NIOSH–233–C] deposits’’ in accordance with the Federal Reserve uses the data collected amendments to Regulation D in the on the FR 2886b to identify present and Hazardous Drugs: Draft NIOSH List of interim final rule published on April 28, potential problems and monitor and Hazardous Drugs in Healthcare 2020 (85 FR 23445). Specifically, the develop a better understanding of Settings, 2020; Procedures; and Risk Board has temporarily revised the activities within the industry. Management Information instructions on the FR Y–9C, Schedule HC–E, items 1(b), 1(c), 2(c) and glossary Legal authorization and AGENCY: Centers for Disease Control and content to remove the transfer or confidentiality: Sections 25 and 25A of Prevention, HHS. withdrawal limit. As a result of the the Federal Reserve Act authorize the ACTION: Notice and request for comment. revision, if a depository institution Federal Reserve to collect the FR 2886b chooses to suspend enforcement of the (12 U.S.C. 602, 625). The obligation to SUMMARY: The National Institute for six transfer limit on a ‘‘savings deposit,’’ report this information is mandatory. -

Efficacy and Safety of Midostaurin-Based Induction and Maintenance Therapy for Newly Diagnosed AML
POST-ASH Issue 4, 2016 Efficacy and Safety of Midostaurin-Based Induction and Maintenance Therapy for Newly Diagnosed AML For more visit ResearchToPractice.com/5MJCASH2016 CME INFORMATION OVERVIEW OF ACTIVITY Each year, thousands of clinicians, basic scientists and other industry professionals sojourn to major international oncology conferences, like the American Society of Hematology (ASH) annual meeting, to hone their skills, network with colleagues and learn about recent advances altering state-of-the-art management in hematologic oncology. These events have become global stages where exciting science, cutting-edge concepts and practice-changing data emerge on a truly grand scale. This massive outpouring of information has enormous benefits for the hematologic oncology community, but the truth is it also creates a major challenge for practicing oncologists and hematologists. Although original data are consistently being presented and published, the flood of information unveiled during a major academic conference is unmatched and leaves in its wake an enormous volume of new knowledge that practicing oncologists must try to sift through, evaluate and consider applying. Unfortunately and quite commonly, time constraints and an inability to access these data sets leave many oncologists struggling to ensure that they’re aware of crucial practice-altering findings. This creates an almost insurmountable obstacle for clinicians in community practice because they are not only confronted almost overnight with thousands of new presentations and