NEW 2018 Specialty Drugs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

September 11, 2018 DUR Minutes
Maine Department of Health and Human Services PAUL R. LEPAGE MaineCare Services BETHANY L. HAMM GOVERNOR Pharmacy Unit ACTING COMMISSIONER 11 State House Station Augusta, Maine 04333-0011 TO: Maine Drug Utilization Review Board DATE: 9/14/2018 RE: Maine DUR Board Meeting minutes from September 11, 2018 ATTENDANCE PRESENT ABSENT EXCUSED Linda Glass, MD X Lisa Wendler, Pharm. D., Clinical Pharmacy Specialist, X Maine Medical CTR Mike Antoniello, MD X Kathleen Polonchek, MD X Kenneth McCall, PharmD X Steve Diaz, MD X Erin Ackley, PharmD. X Corinn Martineau, PharmD. X Non –Voting Mike Ouellette, R.Ph., Change Healthcare X Jeffery Barkin, MD, Change Healthcare X Christopher Pezzullo, State Health Officer DHHS, DO X Jill Kingsbury, MaineCare Pharmacy Director X Guests of the Board: Ed Bosshart, PharmD, Jeff Caulfield, Lead Epidemiologist for Viral Infections from CDC: Discussed HCV treatment. CALL TO ORDER: 5:30PM Jill Kingsbury called the meeting to order at 5:30 PM. PUBLIC COMMENTS Robert Mead from Pfizer: Highlighted the attributes of Retacrit. Jane Guo from Otsuka: Highlighted the attributes of Jynarque. OLD BUSINESS DUR MINUTES The June DUR meeting minutes were accepted as written. MAINECARE UPDATE No update at this time. NEW BUSINESS INTRODUCTION: USE OF CHRONIC TRIPTANS The use of triptans has become standard of care for the treatment of acute migraine headaches, given their effectiveness, safety and tolerability. However, like many medications used to treat migraine, overuse renders them less effective. Additionally, rebound headaches from triptan overuse is common. For patients who experience frequent headaches, or whose headaches are long lasting or chronic, use of headache prophylactic medications are recommended by several medical associations, including the American Headache Society and the American Academy of Neurology. -

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

TEXAS MEDICAID Clinical Edit Prior Authorization Epoetin Alfa (PROCRIT)
TEXAS MEDICAID Clinical Edit Prior Authorization epoetin alfa (PROCRIT) STEP 1: CLEARLY PRINT AND COMPLETE TO EXPEDITE PROCESSING Date: Prescriber First & Last Name: Patient First & Last Name: Prescriber NPI: Patient Address: Prescriber Address: Patient ID: Prescriber Phone: Patient Date of Birth: Prescriber Fax: STEP 2: MEDICATION INFORMATION Medication Requested (Name): Quantity Requested: Dose Requested: Dosing Instructions: Patient’s Primary Diagnosis: ____________________________________ ICD 10 Code: __________ Please indicate ONE (1) of the following: OR STAR / STAR KIDS client (Go to Step 3 - PDL PA Criteria Applies) OR CHIP / PERINATE client (Go to Step 4) STEP 3: PDL PRIOR AUTHORIZATION CRITERIA FOR NON-PREFERRED PRODUCT 1. Has the client failed a 30-day treatment trial with at least 1 preferred agent in the last 180 days? Yes (Go to Step 4 Question 1) No (Go to #2) 2. Is there a documented allergy or contraindication to preferred agents in this class? Yes (Go to Step 4 Question 1) No (Go to #3) 3. Is the drug necessary for treatment of stage-4 advanced metastatic cancer and associated conditions? Yes (Go to Step 4 Question 1) No (Deny) Rev. 11/18/2020 Page 1 of 3 Version 1.5 STEP 4: CLINICAL PRIOR AUTHORIZATION CRITERIA 1. Does the client have a diagnosis of chronic renal failure in the last 730 days? Yes (Go to #7) No (Go to #2) 2. Does the client have a diagnosis of cancer in the last 730 days? Yes (Go to #3) No (Go to #5) 3. Does the client have a history of an antineoplastic agent in the last 30 days? Examples of antineoplastic -

FLT3 Inhibitors in Acute Myeloid Leukemia Mei Wu1, Chuntuan Li2 and Xiongpeng Zhu2*
Wu et al. Journal of Hematology & Oncology (2018) 11:133 https://doi.org/10.1186/s13045-018-0675-4 REVIEW Open Access FLT3 inhibitors in acute myeloid leukemia Mei Wu1, Chuntuan Li2 and Xiongpeng Zhu2* Abstract FLT3 mutations are one of the most common findings in acute myeloid leukemia (AML). FLT3 inhibitors have been in active clinical development. Midostaurin as the first-in-class FLT3 inhibitor has been approved for treatment of patients with FLT3-mutated AML. In this review, we summarized the preclinical and clinical studies on new FLT3 inhibitors, including sorafenib, lestaurtinib, sunitinib, tandutinib, quizartinib, midostaurin, gilteritinib, crenolanib, cabozantinib, Sel24-B489, G-749, AMG 925, TTT-3002, and FF-10101. New generation FLT3 inhibitors and combination therapies may overcome resistance to first-generation agents. Keywords: FMS-like tyrosine kinase 3 inhibitors, Acute myeloid leukemia, Midostaurin, FLT3 Introduction RAS, MEK, and PI3K/AKT pathways [10], and ultim- Acute myeloid leukemia (AML) remains a highly resist- ately causes suppression of apoptosis and differentiation ant disease to conventional chemotherapy, with a me- of leukemic cells, including dysregulation of leukemic dian survival of only 4 months for relapsed and/or cell proliferation [11]. refractory disease [1]. Molecular profiling by PCR and Multiple FLT3 inhibitors are in clinical trials for treat- next-generation sequencing has revealed a variety of re- ing patients with FLT3/ITD-mutated AML. In this re- current gene mutations [2–4]. New agents are rapidly view, we summarized the preclinical and clinical studies emerging as targeted therapy for high-risk AML [5, 6]. on new FLT3 inhibitors, including sorafenib, lestaurtinib, In 1996, FMS-like tyrosine kinase 3/internal tandem du- sunitinib, tandutinib, quizartinib, midostaurin, gilteriti- plication (FLT3/ITD) was first recognized as a frequently nib, crenolanib, cabozantinib, Sel24-B489, G-749, AMG mutated gene in AML [7]. -

Federal Register Notice 5-1-2020 Pdf Icon[PDF – 358
Federal Register / Vol. 85, No. 85 / Friday, May 1, 2020 / Notices 25439 confidential by the respondent (5 U.S.C. schedules. Other than examination DEPARTMENT OF HEALTH AND 552(b)(4)). reports, it provides the only financial HUMAN SERVICES Current actions: The Board has data available for these corporations. temporarily revised the instructions to The Federal Reserve is solely Centers for Disease Control and the FR Y–9C report to accurately reflect responsible for authorizing, supervising, Prevention the revised definition of ‘‘savings and assigning ratings to Edges. The [CDC–2020–0046; NIOSH–233–C] deposits’’ in accordance with the Federal Reserve uses the data collected amendments to Regulation D in the on the FR 2886b to identify present and Hazardous Drugs: Draft NIOSH List of interim final rule published on April 28, potential problems and monitor and Hazardous Drugs in Healthcare 2020 (85 FR 23445). Specifically, the develop a better understanding of Settings, 2020; Procedures; and Risk Board has temporarily revised the activities within the industry. Management Information instructions on the FR Y–9C, Schedule HC–E, items 1(b), 1(c), 2(c) and glossary Legal authorization and AGENCY: Centers for Disease Control and content to remove the transfer or confidentiality: Sections 25 and 25A of Prevention, HHS. withdrawal limit. As a result of the the Federal Reserve Act authorize the ACTION: Notice and request for comment. revision, if a depository institution Federal Reserve to collect the FR 2886b chooses to suspend enforcement of the (12 U.S.C. 602, 625). The obligation to SUMMARY: The National Institute for six transfer limit on a ‘‘savings deposit,’’ report this information is mandatory. -

Efficacy and Safety of Midostaurin-Based Induction and Maintenance Therapy for Newly Diagnosed AML
POST-ASH Issue 4, 2016 Efficacy and Safety of Midostaurin-Based Induction and Maintenance Therapy for Newly Diagnosed AML For more visit ResearchToPractice.com/5MJCASH2016 CME INFORMATION OVERVIEW OF ACTIVITY Each year, thousands of clinicians, basic scientists and other industry professionals sojourn to major international oncology conferences, like the American Society of Hematology (ASH) annual meeting, to hone their skills, network with colleagues and learn about recent advances altering state-of-the-art management in hematologic oncology. These events have become global stages where exciting science, cutting-edge concepts and practice-changing data emerge on a truly grand scale. This massive outpouring of information has enormous benefits for the hematologic oncology community, but the truth is it also creates a major challenge for practicing oncologists and hematologists. Although original data are consistently being presented and published, the flood of information unveiled during a major academic conference is unmatched and leaves in its wake an enormous volume of new knowledge that practicing oncologists must try to sift through, evaluate and consider applying. Unfortunately and quite commonly, time constraints and an inability to access these data sets leave many oncologists struggling to ensure that they’re aware of crucial practice-altering findings. This creates an almost insurmountable obstacle for clinicians in community practice because they are not only confronted almost overnight with thousands of new presentations and -

Disruption of CSF-1R Signaling Inhibits Growth of AML with Inv(16)
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2021 Disruption of CSF-1R signaling inhibits growth of AML with inv(16) Simonis, Alexander ; Russkamp, Norman F ; Mueller, Jan ; Wilk, C Matthias ; Wildschut, Mattheus H E ; Myburgh, Renier ; Wildner-Verhey van Wijk, Nicole ; Mueller, Rouven ; Balabanov, Stefan ; Valk, Peter J M ; Theocharides, Alexandre P A ; Manz, Markus G DOI: https://doi.org/10.1182/bloodadvances.2020003125 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-202789 Journal Article Published Version The following work is licensed under a Publisher License. Originally published at: Simonis, Alexander; Russkamp, Norman F; Mueller, Jan; Wilk, C Matthias; Wildschut, Mattheus H E; Myburgh, Renier; Wildner-Verhey van Wijk, Nicole; Mueller, Rouven; Balabanov, Stefan; Valk, Peter J M; Theocharides, Alexandre P A; Manz, Markus G (2021). Disruption of CSF-1R signaling inhibits growth of AML with inv(16). Blood advances, 5(5):1273-1277. DOI: https://doi.org/10.1182/bloodadvances.2020003125 STIMULUS REPORT Disruption of CSF-1R signaling inhibits growth of AML with inv(16) Alexander Simonis,1,* Norman F. Russkamp,1,* Jan Mueller,1 C. Matthias Wilk,1 Mattheus H. E. Wildschut,1,2 Renier Myburgh,1 Nicole Wildner-Verhey van Wijk,1 Rouven Mueller,1 Stefan Balabanov,1 Peter J. M. Valk,3 Alexandre P. A. Theocharides,1 and Markus G. Manz1 1Department of Medical Oncology and Hematology, University Hospital -

UWHC Guidelines for the Use of Darbepoetin and Epoetin
Use of Darbepoetin and Epoetin in Non-Nephrology Patients – Adult/Pediatric – Inpatient/Ambulatory Clinical Practice Guideline Table of Contents Executive Summary ......................................................................................... 3 Scope ............................................................................................................. 7 Methodology ................................................................................................... 7 Definitions (optional): ...................................................................................... 8 Introduction.................................................................................................... 8 Recommendations........................................................................................... 9 UW Health Implementation............................................................................ 19 References ................................................................................................... 19 Note: Active Table of Contents Click to follow link CPG Contact for Changes: Name: Philip Trapskin, PharmD, BCPS, Manager, DPP Phone Number: 608-263-1328 Email address: [email protected] CPG Contact for Content: Name: Jason Bergsbaken, PharmD Phone Number: 608-265-0341 Email address: [email protected] Copyright © 2015 University of Wisconsin Hospitals and Clinics Authority Contact: [email protected] Vermeulen, [email protected] Last Revised: 07/2015 Guideline Authors: Jason Bergsbaken, PharmD Coordinating -

Dr. Winegarden Presentation
Empowering Market Competition through Biosimilars Presentation to National Council of Insurance Legislators (NCOIL) 2019 Summer Meeting Newport Beach, California July 12, 2019 Total Savings (in millions) 25% 50% 75% Originator Total Annual Savings for Current, 25%, Drug Class Current Biosimilar Biosimilar Biosimilar Biologic 50%, and 75% Biosimilar Share Share Share Share Scenarios Compared to All-Originator Infliximab Remicade $79.4 $318.2 $636.5 $954.7 Biologic Baseline Pegfilgrastim Neulasta $21.8 $121.9 $243.8 $365.7 Filgrastim Neupogen $152.1 $152.1 $152.1 $206.8 Epoetin Alfa Epogen & Procrit $0.5 $8.4 $16.9 $25.3 Bevacizumab Avastin $0.0 $199.2 $398.5 $597.7 Trastuzumab Herceptin $0.0 $208.0 $415.9 $623.9 • Biosimilars price discounts expected 30% - Rituxumab Rituxan $0.0 $280.6 $561.2 $841.8 40% off originator biologic Etanercept Enbrel $0.0 $324.0 $648.0 $972.1 • Forthcoming study estimates possible Adalimunab Humira $0.0 $861.1 $1,722.1 $2,583.2 health care savings from wider adoption of biosimilars. GRAND TOTAL $253.8 $2,473.6 $4,795.0 $7,171.2 • Estimates are based on the average sales price (ASP) data that are effective from April 2019 through June 2019 and rolling 12-month volume data through February 2019. Biosimilars have no “clinically meaningful difference” in safety, purity, • Methodology: compare potential savings to hypothetical all-originator biologic scenario and effectiveness relative to its reference originator biologic. Just like • Over 10 years, potential savings of $24.7 generic medicines, the benefits of biosimilars are the substantial price billion, $48.0 billion, and $71.7 billion respectively. -
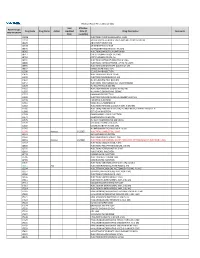
2021 Prior Authorization List Part B Appendix a (PDF)
Medicare Part B PA List Effective 2021 Last Effective Part B Drugs: Drug Code Drug Name Action Updated Date (if Drug Description Comments STEP THERAPY Date available) C9050 INJECTION, EMAPALUMAB-LZSG, 1 MG C9122 MOMETASONE FUROATE SINUS IMPLANT 10 MCG SINUVA J0129 ABATACEPT INJECTION J0178 AFLIBERCEPT INJECTION J0570 BUPRENORPHINE IMPLANT 74.2MG J0585 INJECTION,ONABOTULINUMTOXINA J0717 CERTOLIZUMAB PEGOL INJ 1MG J0718 CERTOLIZUMAB PEGOL INJ J0791 INJECTION CRIZANLIZUMAB-TMCA 5 MG J0800 INJECTION, CORTICOTROPIN, UP TO 40 UNITS J0896 INJECTION LUSPATERCEPT-AAMT 0.25 MG J0897 DENOSUMAB INJECTION J1300 ECULIZUMAB INJECTION J1428 INJECTION ETEPLIRSEN 10 MG J1429 INJECTION GOLODIRSEN 10 MG J1442 INJ FILGRASTIM EXCL BIOSIMIL J1447 INJECTION, TBO-FILGRASTIM, 1 MICROGRAM J1459 INJ IVIG PRIVIGEN 500 MG J1555 INJECTION IMMUNE GLOBULIN 100 MG J1556 INJ, IMM GLOB BIVIGAM, 500MG J1557 GAMMAPLEX INJECTION J1558 INJECTION IMMUNE GLOBULIN XEMBIFY 100 MG J1559 HIZENTRA INJECTION J1561 GAMUNEX-C/GAMMAKED J1562 INJECTION; IMMUNE GLOBULIN 10%, 5 GRAMS J1566 INJECTION, IMMUNE GLOBULIN, INTRAVENOUS, LYOPHILIZED (E.G. P J1568 OCTAGAM INJECTION J1569 GAMMAGARD LIQUID INJECTION J1572 FLEBOGAMMA INJECTION J1575 INJ IG/HYALURONIDASE 100 MG IG J1599 IVIG NON-LYOPHILIZED, NOS J1602 GOLIMUMAB FOR IV USE 1MG J1745 INJ INFLIXIMAB EXCL BIOSIMILR 10 MG J1930 Remove 1/1/2021 INJECTION, LANREOTIDE, 1 MG J2323 NATALIZUMAB INJECTION J2350 INJECTION OCRELIZUMAB 1 MG J2353 Remove 1/1/2021 INJECTION, OCTREOTIDE, DEPOT FORM FOR INTRAMUSCULAR INJECTION, 1 MG J2357 INJECTION, OMALIZUMAB, -

Presentation Title
The COMMANDS trial: a phase 3 study of the efficacy and safety of luspatercept versus epoetin alfa for the treatment of anemia due to Revised International Prognostic Scoring System Very Low-, Low-, or Intermediate-risk myelodysplastic syndromes in erythropoiesis stimulating agent-naive patients who require red blood cell transfusions Matteo Della Porta,1,2 Uwe Platzbecker,3 Valeria Santini,4 Guillermo Garcia-Manero,5 Rami S. Komrokji,6 Rodrigo Ito,7 Pierre Fenaux8 1Cancer Center IRCCS Humanitas Research Hospital, Milan, Italy; 2Department of Biomedical Sciences, Humanitas University, Milan, Italy; 3Medical Clinic and Policlinic 1, Hematology and Cellular Therapy, University Hospital Leipzig, Leipzig, Germany; 4Azienda Ospedaliero-Universitaria Careggi, University of Florence, Florence, Italy; 5Department of Leukemia, University of Texas MD Anderson Cancer Center, Houston, TX; 6Moffitt Cancer Center, Tampa, FL; 7Bristol Myers Squibb, Princeton, NJ; 8Service d'Hématologie Séniors, Hôpital Saint-Louis, Université Paris 7, Paris, France Presentation 2198 Presenting author disclosures M.D.P.: no conflicts of interest to disclose. 2 Introduction and objectives Introduction • Studies of epoetin alfa and darbepoetin alfa have demonstrated efficacy among patients with LR-MDS, but the patient population in which a clinically significant effect is observed may be limited1,2 • Luspatercept, a first-in-class erythroid maturation agent with a mechanism of action distinct from ESAs,3 is approved by the US FDA for the treatment of anemia failing an -

Recommendations from York and Scarborough Medicines
Recommendations from York and Scarborough Medicines Commissioning Committee July 2018 Drug name Indication Recommendation, rationale and place in RAG status Potential full year cost impact therapy CCG commissioned Technology Appraisals 1. Nil NHSE commissioned Technology Appraisals – for noting 2. TA520: Atezolizumab for Atezolizumab is recommended as an option for Red No cost impact to CCGs as NHS England treating locally advanced or treating locally advanced or metastatic non- commissioned. metastatic non-small-cell lung small-cell lung cancer (NSCLC) in adults who cancer after chemotherapy have had chemotherapy (and targeted treatment if they have an EGFR- or ALK‑ positive tumour), only if: atezolizumab is stopped at 2 years of uninterrupted treatment or earlier if the disease progresses and the company provides atezolizumab with the discount agreed in the patient access scheme. 3. TA522: Pembrolizumab for Pembrolizumab is recommended for use within Red No cost impact to CCGs as NHS England untreated locally advanced or the Cancer Drugs Fund as an option for commissioned. metastatic urothelial cancer untreated locally advanced or metastatic when cisplatin is unsuitable urothelial carcinoma in adults when cisplatin- containing chemotherapy is unsuitable, only if: pembrolizumab is stopped at 2 years of uninterrupted treatment or earlier if the disease progresses and the conditions of the managed access agreement for pembrolizumab are followed TA523: Midostaurin for Midostaurin is recommended, within its Red No cost impact to CCGs as NHS England untreated acute myeloid marketing authorisation, as an option in adults commissioned. leukaemia for treating newly diagnosed acute FLT3- mutation-positive myeloid leukaemia with standard daunorubicin and cytarabine as induction therapy, with high-dose cytarabine as consolidation therapy, and alone after complete response as maintenance therapy.