Epithermal Neutron Activation Analysis of Short-Lived Radionuclides Using a Pneumatic Transport System and a Pulsed D-T Neutron Generator
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
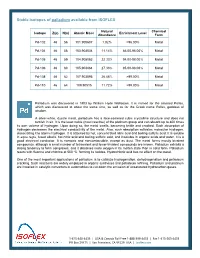
Stable Isotopes of Palladium Available from ISOFLEX
Stable isotopes of palladium available from ISOFLEX Natural Chemical Isotope Z(p) N(n) Atomic Mass Enrichment Level Abundance Form Pd-102 46 56 101.905607 1.02% >96.00% Metal Pd-104 46 58 103.904034 11.14% 86.00-98.00% Metal Pd-105 46 59 104.905083 22.33% 94.00-98.00% Metal Pd-106 46 60 105.903484 27.33% 95.00-98.00% Metal Pd-108 46 62 107.903895 26.46% >99.00% Metal Pd-110 46 64 109.90515 11.72% >99.00% Metal Palladium was discovered in 1803 by William Hyde Wollaston. It is named for the asteroid Pallas, which was discovered at about the same time, as well as for the Greek name Pallas, goddess of wisdom. A silver-white, ductile metal, palladium has a face-centered cubic crystalline structure and does not tarnish in air. It is the least noble (most reactive) of the platinum group and can absorb up to 800 times its own volume of hydrogen. Upon doing so, the metal swells, becoming brittle and cracked. Such absorption of hydrogen decreases the electrical conductivity of the metal. Also, such absorption activates molecular hydrogen, dissociating it to atomic hydrogen. It is attacked by hot, concentrated nitric acid and boiling sulfuric acid. It is soluble in aqua regia, fused alkalis, hot nitric acid and boiling sulfuric acid, and insoluble in organic acids and water. It is a good electrical conductor. It is nontoxic and noncombustible, except as dust. The metal forms mostly bivalent compounds, although a small number of tetravalent and fewer trivalent compounds are known. -

Measurement and Analysis of the Resolved Resonance Cross Sections of the Natural Hafnium Isotopes
CORE Metadata, citation and similar papers at core.ac.uk Provided by University of Birmingham Research Archive, E-theses Repository MEASUREMENT AND ANALYSIS OF THE RESOLVED RESONANCE CROSS SECTIONS OF THE NATURAL HAFNIUM ISOTOPES by TIMOTHY CHRISTOPHER WARE A thesis submitted to The University of Birmingham for the degree of DOCTOR OF PHILOSOPHY School of Physics and Astronomy College of Engineering and Physical Sciences The University of Birmingham June 2010 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. ABSTRACT Hafnium is a ductile metallic element with a large neutron absorption cross section. It can be used in reactor control rods to regulate the fission process. The NEA High Priority Request List for nuclear data presents a need for improved characterisation of the hafnium cross section in the resolved resonance region. This thesis presents new resonance cross section parameters for the six natural hafnium isotopes. Cross section measurements, supported by the NUDAME and EUFRAT projects, were performed at the IRMM Geel GELINA time-of-flight facility. Capture experiments were conducted on the 12 m, 28 m and 58 m flight paths using C6D6 detectors and transmission experiments were performed at flight paths of 26 m and 49 m using a 6Li glass detector. -

First Search for $\Alpha $ Decays of Naturally Occurring Hf Nuclides With
First search for α decays of naturally occurring Hf nuclides with emission of γ quanta F.A. Danevicha,1, M. Hultb, D.V. Kasperovycha, G.P. Kovtunc,d, K.V. Kovtune, G. Lutterb, G. Marissensb, O.G. Polischuka, S.P. Stetsenkoc, V.I. Tretyaka aInstitute for Nuclear Research, 03028 Kyiv, Ukraine bEuropean Commission, Joint Research Centre, Retieseweg 111, 2440 Geel, Belgium cNational Scientific Center Kharkiv Institute of Physics and Technology, 61108 Kharkiv, Ukraine dKarazin Kharkiv National University, 61022 Kharkiv, Ukraine ePublic Enterprise “Scientific and Technological Center Beryllium”, 61108 Kharkiv, Ukraine Abstract The first ever search for α decays to the first excited state in Yb was performed for six isotopes of hafnium (174, 176, 177, 178, 179, 180) using a high purity Hf-sample of natural isotopic abundance with a mass of 179.8 g. For 179Hf, also α decay to the ground state of 175Yb was searched for thanks to the β-instability of the daughter nuclide 175Yb. The measurements were conducted using an ultra low-background HPGe-detector system located 225 m underground. After 75 d of data taking no decays were detected but lower 15 18 bounds for the half-lives of the decays were derived on the level of lim T1/2 ∼ 10 −10 a. The decay with the shortest half-life based on theoretical calculation is the decay of 174Hf to the first 2+ 84.3 keV excited level of 170Yb. The experimental lower bound was found 15 to be T1/2 ≥ 3.3 × 10 a. Keywords: Alpha decay; 174Hf, 176Hf, 177Hf, 178Hf, 179Hf, 180Hf, Low-background HPGe γ spec- trometry arXiv:1911.02597v1 [nucl-ex] 6 Nov 2019 1 INTRODUCTION Alpha decay is one of the most important topics of nuclear physics both from the theoretical and experimental points of view. -

(12) United States Patent (10) Patent No.: US 8,625,731 B2 Holden Et Al
USOO8625731B2 (12) United States Patent (10) Patent No.: US 8,625,731 B2 Holden et al. (45) Date of Patent: Jan. 7, 2014 (54) COMPACT NEUTRONGENERATOR FOR (51) Int. Cl. MEDICAL AND COMMERCIAL SOTOPE G2G 4/02 (2006.01) PRODUCTION, FISSION PRODUCT G2G 4/00 (2006.01) PURIFICATION AND CONTROLLED (52) U.S. Cl. GAMMA REACTIONS FOR DIRECT USPC ............................ 376/108: 376/112:376/156 ELECTRIC POWER GENERATION (58) Field of Classification Search USPC .......................... 376/157, 108,156, 171, 172 (76) Inventors: Charles S. Holden, San Francisco, CA See application file for complete search history. (US); Robert E. Schenter, Portland, OR (US) (56) References Cited *) Notice: Subject to anyy disclaimer, the term of this U.S. PATENT DOCUMENTS patent is extended or adjusted under 35 3,748,226 A * 7/1973 Ribe et al. ..................... 376,124 U.S.C. 154(b) by 961 days. 3,778,627 A * 12/1973 Carpenter ...... ... 376, 192 4,749,540 A * 6/1988 Bogartet al. .. ... 376/133 (21) Appl. No.: 12/296,844 4,997,619 A * 3/1991 Pettus ............ ... 376,288 2004/02284.33 A1* 1 1/2004 Magill et al. ... 376/347 (22) PCT Filed: Apr. 13, 2007 2005/022O248 A1* 10, 2005 Ritter ...... ... 376/190 2008/O144762 A1* 6/2008 Holden et al. ..... ... 376/416 (86). PCT No.: PCT/US2OOTAO66668 * cited by examiner S371 (c)(1), (2), (4) Date: Oct. 10, 2008 Primary Examiner — Jack W Keith Assistant Examiner — Sean P Burke (87) PCT Pub. No.: WO2008/060663 (74) Attorney, Agent, or Firm — Craig M. Stainbrook; PCT Pub. Date: May 22, 2008 Stainbrook & Stainbrook, LLP (65) Prior Publication Data (57) ABSTRACT A neutron generator and isotope production apparatus and US 2011 FO268237 A1 Nov. -

4.62 Samarium
IUPAC 4.62 samarium Stable Relative Mole isotope atomic mass fraction 144 Sm 143.912 01 0.0308 147 † Sm 146.914 90 0.1500 148 Sm † 147.914 83 0.1125 149 Sm 148.917 19 0.1382 150 Sm 149.917 28 0.0737 152 Sm 151.919 74 0.2674 154 Sm 153.922 22 0.2274 † Radioactive isotope having a relatively long half-life and a characteristic terrestrial isotopic composition that contributes significantly and reproducibly to the determination of the standard atomic weight of the element in normal materials . The half-lives of 147 Sm and 148 Sm are 1.06 × 10 11 years and 7 × 10 15 years, respectively. 4.62.1 Samarium isotopes in Earth/planetary science One possible origin for the Moon is from debris ejected by an indirect giant impact of Earth by an astronomical body the size of Mars when the Earth was forming [433]. The kinetic energy liberated is thought to have melted a large part of the Moon forming a lunar magma ocean. P.O. 13757, Research Triangle Park, NC (919) 485-8700 IUPAC Samarium isotope measurement results [434], along with measurements of isotopes of hafnium, tungsten, and neodymium[435], suggest that lunar magma formed about 70 × 10 6 years after the Solar System formed and had crystallized by about 215 × 10 6 years after formation. 147 Sm is used to study the formation of potassium, rare earth elements , and phosphorus-rich rocks [436]. 4.62.2 Samarium isotopes in geochronology 147 Sm is used for determining formation ages of igneous and metamorphic rocks via analysis of the minerals which compose them, such as those shown in Figure 4.62.1 [437-439]. -

The Discoverers of the Ruthenium Isotopes
•Platinum Metals Rev., 2011, 55, (4), 251–262• The Discoverers of the Ruthenium Isotopes Updated information on the discoveries of the six platinum group metals to 2010 http://dx.doi.org/10.1595/147106711X592448 http://www.platinummetalsreview.com/ By John W. Arblaster This review looks at the discovery and the discoverers Wombourne, West Midlands, UK of the thirty-eight known ruthenium isotopes with mass numbers from 87 to 124 found between 1931 and 2010. Email: [email protected] This is the sixth and fi nal review on the circumstances surrounding the discoveries of the isotopes of the six platinum group elements. The fi rst review on platinum isotopes was published in this Journal in October 2000 (1), the second on iridium isotopes in October 2003 (2), the third on osmium isotopes in October 2004 (3), the fourth on palladium isotopes in April 2006 (4) and the fi fth on rhodium isotopes in April 2011 (5). An update on the new isotopes of palladium, osmium, iridium and platinum discovered since the previous reviews in this series is also included. Naturally Occurring Ruthenium Of the thirty-eight known isotopes of ruthenium, seven occur naturally with the authorised isotopic abun- dances (6) shown in Table I. The isotopes were fi rst detected in 1931 by Aston (7, 8) using a mass spectrograph at the Cavendish Laboratory, Cambridge University, UK. Because of diffi cult experimental conditions due to the use of poor quality samples, Aston actually only detected six of the isotopes and obtained very approximate Table I The Naturally Occurring Isotopes of Ruthenium Mass number Isotopic Abundance, % 96Ru 5.54 98Ru 1.87 99Ru 12.76 100Ru 12.60 101Ru 17.06 102Ru 31.55 104Ru 18.62 251 © 2011 Johnson Matthey http://dx.doi.org/10.1595/147106711X592448 •Platinum Metals Rev., 2011, 55, (4)• percentage abundances. -

Zerohack Zer0pwn Youranonnews Yevgeniy Anikin Yes Men
Zerohack Zer0Pwn YourAnonNews Yevgeniy Anikin Yes Men YamaTough Xtreme x-Leader xenu xen0nymous www.oem.com.mx www.nytimes.com/pages/world/asia/index.html www.informador.com.mx www.futuregov.asia www.cronica.com.mx www.asiapacificsecuritymagazine.com Worm Wolfy Withdrawal* WillyFoReal Wikileaks IRC 88.80.16.13/9999 IRC Channel WikiLeaks WiiSpellWhy whitekidney Wells Fargo weed WallRoad w0rmware Vulnerability Vladislav Khorokhorin Visa Inc. Virus Virgin Islands "Viewpointe Archive Services, LLC" Versability Verizon Venezuela Vegas Vatican City USB US Trust US Bankcorp Uruguay Uran0n unusedcrayon United Kingdom UnicormCr3w unfittoprint unelected.org UndisclosedAnon Ukraine UGNazi ua_musti_1905 U.S. Bankcorp TYLER Turkey trosec113 Trojan Horse Trojan Trivette TriCk Tribalzer0 Transnistria transaction Traitor traffic court Tradecraft Trade Secrets "Total System Services, Inc." Topiary Top Secret Tom Stracener TibitXimer Thumb Drive Thomson Reuters TheWikiBoat thepeoplescause the_infecti0n The Unknowns The UnderTaker The Syrian electronic army The Jokerhack Thailand ThaCosmo th3j35t3r testeux1 TEST Telecomix TehWongZ Teddy Bigglesworth TeaMp0isoN TeamHav0k Team Ghost Shell Team Digi7al tdl4 taxes TARP tango down Tampa Tammy Shapiro Taiwan Tabu T0x1c t0wN T.A.R.P. Syrian Electronic Army syndiv Symantec Corporation Switzerland Swingers Club SWIFT Sweden Swan SwaggSec Swagg Security "SunGard Data Systems, Inc." Stuxnet Stringer Streamroller Stole* Sterlok SteelAnne st0rm SQLi Spyware Spying Spydevilz Spy Camera Sposed Spook Spoofing Splendide -

Study of the Properties of Hydrogen and Deuterium in Beta Phase Palladium Hydride and Deuteride
Study of the Properties of Hydrogen and Deuterium in Beta Phase Palladium Hydride and Deuteride SIMON ANTHONY STEEL 18/04/2018 Materials and Physics Research Group School of Computing Science & Engineering This thesis is submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy Dedication This work is dedicated to the memory of Professor Donald Keith Ross. “I may not have gone where I intended to go, but I think I have ended up where I needed to be.” Douglas Adams i Contents Dedication .................................................................................................................................... i Figures ...................................................................................................................................... vii Acknowledgements .................................................................................................................... x Glossary .................................................................................................................................... xii Units, Constants, and Standard Values ................................................................................... xiii Conventions in this Document ................................................................................................ xiv Abstract .................................................................................................................................... xv 1 Introduction ........................................................................................................................ -

The Discoverers of the Palladium Isotopes the THIRTY-FOUR KNOWN PALLADIUM ISOTOPES FOUND BETWEEN 1935 and 1997
DOI: 10.1595/147106706X110817 The Discoverers of the Palladium Isotopes THE THIRTY-FOUR KNOWN PALLADIUM ISOTOPES FOUND BETWEEN 1935 AND 1997 By J. W. Arblaster Coleshill Laboratories, Gorsey Lane, Coleshill, West Midlands B46 1JU, U.K.; E-mail: [email protected] This is the fourth in a series of reviews of circumstances surrounding the discoveries of the isotopes of the six platinum group elements. The first review, on platinum isotopes, was published in this Journal in October 2000, the second, on iridium isotopes, was published here in October 2003 and the third, on osmium isotopes, was published in October 2004 (1). The current review looks at the discovery and the discoverers of the thirty-four isotopes of palladium. Of the thirty-four known isotopes of palladium, ment activities found for palladium, such as a half- six occur naturally with the following authorised life of six hours discovered by Fermi et al. in 1934 isotopic abundances (2): (9) and half-lifes of 3 minutes and 60 hours discov- ered by Kurchatov et al. (10) in 1935 do not appear to have been confirmed. The Naturally Occurring Isotopes of Palladium In 1940, Nishima et al. (11) obtained an unspec- Mass number Isotopic abundance, % ified activity with a half-life of 26 minutes which is also likely to have been 111Pd. The actual half-life of 102Pd 1.02 111 104Pd 11.14 Pd is now known to be 23 minutes, so the differ- 105Pd 22.33 ent values obtained above are probably indicative 106 Pd 27.33 of calibration problems. 108 Pd 26.46 These unspecified activities raise problems con- 110Pd 11.72 cerning the precedence for treating each discovery in this paper. -

110 Journal of New Energy Vol. 2, No 2
110 Journal of New Energy Vol. 2, no 2 OPERATING THE LENT-1 TRANSMUTATION REACTOR: A PRELIMINARY REPORT By Hal Fox and Shang-Xian Jin ABSTRACT The Low-Energy Nuclear Transmutation (LENT-1) reactor can transmute thorium into smaller mass elements. This transmutation process differs markedly from the natural decay of thorium-232 into lead-208. Using a small amount of thorium nitrate dissolved in distilled water as the electrolyte, the LENT-1 reactor will transmute essentially all of the thorium into small mass elements in thirty minutes processing time. Considerable development work is required to understand the role of reactor parameters in producing various transmuted smaller mass elements. A. INTRODUCTION Most of the current models of nuclear reactions require that high energy be used to cause nuclear reactions, except for the decay of naturally radioactive substances such as thorium and uranium. Nearly all of the nuclear experimental data has been obtained by experiments based on nuclear reactors or using high energy particle accelerators. The study of nuclear reactions in or on the surface of a metal lattice is relatively new. Two international conferences on Low-Energy Nuclear Reactions have been held and the proceedings published in the Journal of New Energy [1,2]. Several important papers have reported on experiments in which low-energy nuclear reactions are observed. This paper reports on the results that have been achieved by various workers using the Low-Energy Nuclear Transmutation (LENT-1) reactor. The LENT-1 reactor consists of a cylindrical electrode and a disk-shaped electrode positioned on the interior of the cylindrical electrode. -

A Modified Nuclear Model for Binding Energy of Nuclei And
A MODIFIED NUCLEAR MODEL FOR BINDING ENERGY OF NUCLEI AND THE ISLAND OF STABILITY BY KENNETH KIPCHUMBA SIRMA A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE AWARD OF THE DEGREE OF DOCTOR OF PHILOSOPHY IN PHYSICS, UNIVERSITY OF ELDORET, KENYA. MAY, 2021 ii DECLARATION Declaration by the Candidate I declare that this is my original and personal work and has not been presented for a degree in any other university. This thesis is not to be reproduced without the prior written permission of the author and/or University of Eldoret. Kenneth Kipchumba Sirma ______________________________ _______________________ SC/PHD/002/15 Date Approval by Supervisors This thesis has been submitted for examination with our approval as University Supervisors. ______________________________ _______________________ Prof. Kapil M. Khanna Date Department of Physics University of Eldoret, Kenya. ______________________________ _______________________ Dr. Samuel L. Chelimo Date Physics Department University of Eldoret, Kenya. iii DEDICATION To my beloved mum Grace for her unconditional love, advice and support. To our children Gael and Abby you are blessings. My wife Jacinta, for her love and care. iv ABSTRACT A new nuclear model of quantifying binding energy of nuclei is proposed. The nucleus is assumed to be composed of two regions; the inner core region and surface region. The inner core is assumed to be composed of Z proton-neutron pairs (Z=N) and the surface region is composed of the unpaired neutrons for a nucleus in which N>Z. The interaction between the core and neutrons in the surface region is assumed to be such that it leads to an average potential Vo in which each neutron in the surface region can move. -

On-Line Separation of Short-Lived Tungsten Isotopes from Tantalum; Hafnium and Lutetium by Adsorption on Ion Exchangers from Aqueous Ammonia Solution
Jointly I,ublished by Elsevier Scie.ce S. A~. Lausanne and J.Radioanal.Nucl.Chem.,Letters Akad?miai Kiod6, Bltdapest 214 (I) I-7 (I 996) ON-LINE SEPARATION OF SHORT-LIVED TUNGSTEN ISOTOPES FROM TANTALUM; HAFNIUM AND LUTETIUM BY ADSORPTION ON ION EXCHANGERS FROM AQUEOUS AMMONIA SOLUTION 1 1 1 1 D. Schumann , R. Dressler , St. Taut , H. Nitsche , Z. Szeglowski2, B. Kubica2~ L.I. Guseva 3, 4 G.S. Tikhomirova3, A. Yakushev~, O. Constantinescu , V.P. Domanov 4, M. Constantinescu 4, Dinh Thi Lien 4, Yu. Ts. Oganessian 4, V.B. Brudanin 4, I. Zvara 4, H. Bruchertseifer 5 I Institute of Analytical Chemistry, University of Technology Dresden, 01062 Dresden, Germany 2H. Niewodniczanski Institut of Nuclear Physics, Krakow, Poland ~Institute of Geochemistry and Analytical Chemistry, Moscow, Russia 4joint Institute of Nuclear Research, Dubna, Russia 5paul-Scherrer-Institute, Villigen, Switzerland Received 17 June 1996 Accepted I July 1996 The title goal was achieved using a DOWEX 50Wx8 cation exchange column saturated with La(OH) 3 and ammonia solution as eluent.>Hf, Ta and Lu were adsorbed on this column, where- as W remained in the solution. This chemical system may be used for fast on-line separa- tions of element 106. INTRODUCT ION Subgroup VI elements form oxo-anions in alkaline solu- tion I , whereas subgroup IV and V elements and lanthanides hydroiyze under these conditions. This might be of inter- 0236 -5 731/76/.[/S ~ J 2,0 Cops I"ight ~'9~6 Ak~ch~nlirli KicaM, Blldapr All t il.,ht$ rest'tied SCHUMANN et al.: ON-LINE SEPARATION OF TUNGSTEN ISOTOPES est for fast on-line separation of element 106 from heavy actinides and element 104 produced simultaneously in heavy ion reactions.