The Oxygen Group (Chalcogens)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
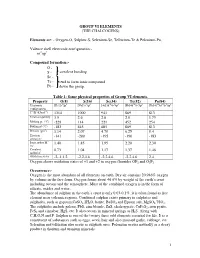
Group Vi Elements (The Chalcogens)
GROUP VI ELEMENTS (THE CHALCOGENS) Elements are: - Oxygen-O, Sulphur-S, Selenium-Se, Tellurium-Te & Polonium-Po. Valence shell electronic configuration:- ns2np4 Compound formation:- O - S - covalent bonding Se - Te - tend to form ionic compound Po - down the group. Table 1: Some physical properties of Group VI elements. Property O(8) S(16) Se(34) Te(52) Po(84) Electronic [He]2s22p4 [Ne]3s23p4 [Ar]3d104s24p4 [Kr]4d105s25p4 [Xe]4f145d106s26p4 configuration 1st IE (kJmol-1) 1314 1000 941 869 813 Electronegativity 3.5 2.6 2.6 2.0 1.75 Melting pt. (oC) -229 114 221 452 254 Boiling pt (oC) -183 445 685 869 813 Density (gm-3) 1.14 2.07 4.79 6.25 9.4 Electron -141 -200 -195 -190 -183 affinity,E- Ionic radius M2- 1.40 1.85 1.95 2.20 2.30 /Ao Covalent 0.73 1.04 1.17 1.37 1.46 radius/Ao Oxidation states -2,-1,1,2 -2,2,4,6 -2,2,4,6 -2,2,4,6 2,4 Oxygen shows oxidation states of +1 and +2 in oxygen fluorides OF2 and O2F2 Occurrence:- Oxygen is the most abundant of all elements on earth. Dry air contains 20.946% oxygen by volume in the free form. Oxygen forms about 46.6% by weight of the earth’s crust including oceans and the atmosphere. Most of the combined oxygen is in the form of silicate, oxides and water. The abundance of sulphur in the earth’s crust is only 0.03-0.1%. it is often found as free element near volcanic regions. -

An Alternate Graphical Representation of Periodic Table of Chemical Elements Mohd Abubakr1, Microsoft India (R&D) Pvt
An Alternate Graphical Representation of Periodic table of Chemical Elements Mohd Abubakr1, Microsoft India (R&D) Pvt. Ltd, Hyderabad, India. [email protected] Abstract Periodic table of chemical elements symbolizes an elegant graphical representation of symmetry at atomic level and provides an overview on arrangement of electrons. It started merely as tabular representation of chemical elements, later got strengthened with quantum mechanical description of atomic structure and recent studies have revealed that periodic table can be formulated using SO(4,2) SU(2) group. IUPAC, the governing body in Chemistry, doesn‟t approve any periodic table as a standard periodic table. The only specific recommendation provided by IUPAC is that the periodic table should follow the 1 to 18 group numbering. In this technical paper, we describe a new graphical representation of periodic table, referred as „Circular form of Periodic table‟. The advantages of circular form of periodic table over other representations are discussed along with a brief discussion on history of periodic tables. 1. Introduction The profoundness of inherent symmetry in nature can be seen at different depths of atomic scales. Periodic table symbolizes one such elegant symmetry existing within the atomic structure of chemical elements. This so called „symmetry‟ within the atomic structures has been widely studied from different prospects and over the last hundreds years more than 700 different graphical representations of Periodic tables have emerged [1]. Each graphical representation of chemical elements attempted to portray certain symmetries in form of columns, rows, spirals, dimensions etc. Out of all the graphical representations, the rectangular form of periodic table (also referred as Long form of periodic table or Modern periodic table) has gained wide acceptance. -

Unit 3 Notes: Periodic Table Notes John Newlands Proposed an Organization System Based on Increasing Atomic Mass in 1864
Unit 3 Notes: Periodic Table Notes John Newlands proposed an organization system based on increasing atomic mass in 1864. He noticed that both the chemical and physical properties repeated every 8 elements and called this the ____Law of Octaves ___________. In 1869 both Lothar Meyer and Dmitri Mendeleev showed a connection between atomic mass and an element’s properties. Mendeleev published first, and is given credit for this. He also noticed a periodic pattern when elements were ordered by increasing ___Atomic Mass _______________________________. By arranging elements in order of increasing atomic mass into columns, Mendeleev created the first Periodic Table. This table also predicted the existence and properties of undiscovered elements. After many new elements were discovered, it appeared that a number of elements were out of order based on their _____Properties_________. In 1913 Henry Mosley discovered that each element contains a unique number of ___Protons________________. By rearranging the elements based on _________Atomic Number___, the problems with the Periodic Table were corrected. This new arrangement creates a periodic repetition of both physical and chemical properties known as the ____Periodic Law___. Periods are the ____Rows_____ Groups/Families are the Columns Valence electrons across a period are There are equal numbers of valence in the same energy level electrons in a group. 1 When elements are arranged in order of increasing _Atomic Number_, there is a periodic repetition of their physical and chemical -

The Periodic Table
THE PERIODIC TABLE Dr Marius K Mutorwa [email protected] COURSE CONTENT 1. History of the atom 2. Sub-atomic Particles protons, electrons and neutrons 3. Atomic number and Mass number 4. Isotopes and Ions 5. Periodic Table Groups and Periods 6. Properties of metals and non-metals 7. Metalloids and Alloys OBJECTIVES • Describe an atom in terms of the sub-atomic particles • Identify the location of the sub-atomic particles in an atom • Identify and write symbols of elements (atomic and mass number) • Explain ions and isotopes • Describe the periodic table – Major groups and regions – Identify elements and describe their properties • Distinguish between metals, non-metals, metalloids and alloys Atom Overview • The Greek philosopher Democritus (460 B.C. – 370 B.C.) was among the first to suggest the existence of atoms (from the Greek word “atomos”) – He believed that atoms were indivisible and indestructible – His ideas did agree with later scientific theory, but did not explain chemical behavior, and was not based on the scientific method – but just philosophy John Dalton(1766-1844) In 1803, he proposed : 1. All matter is composed of atoms. 2. Atoms cannot be created or destroyed. 3. All the atoms of an element are identical. 4. The atoms of different elements are different. 5. When chemical reactions take place, atoms of different elements join together to form compounds. J.J.Thomson (1856-1940) 1. Proposed the first model of the atom. 2. 1897- Thomson discovered the electron (negatively- charged) – cathode rays 3. Thomson suggested that an atom is a positively- charged sphere with electrons embedded in it. -

Development of a Solvent Extraction Process for Group Actinide Recovery from Used Nuclear Fuel
THESIS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Development of a Solvent Extraction Process for Group Actinide Recovery from Used Nuclear Fuel EMMA H. K. ANEHEIM Department of Chemical and Biological Engineering CHALMERS UNIVERSITY OF TECHNOLOGY Gothenburg, Sweden, 2012 Development of a Solvent Extraction Process for Group Actinide Recovery from Used Nuclear Fuel EMMA H. K. ANEHEIM ISBN 978-91-7385-751-2 © EMMA H. K. ANEHEIM, 2012. Doktorsavhandlingar vid Chalmers tekniska högskola Ny serie Nr 3432 ISSN 0346-718X Department of Chemical and Biological Engineering Chalmers University of Technology SE-412 96 Gothenburg Sweden Telephone + 46 (0)31-772 1000 Cover: Radiotoxicity as a function of time for the once through fuel cycle (left) compared to one P&T cycle using the GANEX process (right) (efficiencies: partitioning from Table 5.5.4, transmutation: 99.9%). Calculations performed using RadTox [HOL12]. Chalmers Reproservice Gothenburg, Sweden 2012 Development of a Solvent Extraction Process for Group Actinide Recovery from Used Nuclear Fuel EMMA H. K. ANEHEIM Department of Chemical and Biological Engineering Chalmers University of Technology Abstract When uranium is used as fuel in nuclear reactors it both undergoes neutron induced fission as well as neutron capture. Through successive neutron capture and beta decay transuranic elements such as neptunium, plutonium, americium and curium are produced in substantial amounts. These radioactive elements are mostly long-lived and contribute to a large portion of the long term radiotoxicity of the used nuclear fuel. This radiotoxicity is what makes it necessary to isolate the used fuel for more than 100,000 years in a final repository in order to avoid harm to the biosphere. -

The Group 1A and Group 2A Elements
Cotton chapter 10,11 Group 1A Group 1A Qualitative alkali metal analysis Alkali Metals y The group 1A elements with their ns1 valence electron configurations are very active metals. They lose their valence electrons very readily. They have low ionization energies and react with nonmetals to form ionic solids. 2Na(s) +Cl2(g) Æ 2NaCl(s) y The expected trend in reducing ability, Cs>Rb>K>Na>Li y Alkali metals all react vigorously with water to release hydrogen gas. + ‐ 2M(s) +2H2O(l) Æ 2M (aq) +2OH(aq) +H2(g) y Observed reducing abilities: Li>K>Na First ionization energy Soda production Properties and Trends in Group 1A y The Group 1A metals exhibit regular trends for a number of properties. y Irregular trends suggest that factors are working against each other in determining a property (such as the density “discrepancy” between sodium and potassium). y The alkali metals have two notable physical properties: they are all soft and have low melting points. y When freshly cut, the alkali metals are bright and shiny—typical metallic properties. The metals quickly tarnish, however, as they react with oxygen in the air. Alkali Metal Oxides In the presence of ample oxygen, 4Li + O2 → 2Li2O(regularoxide) 2Na + O2 → Na2O2 (peroxide) K+O2 → KO2 (superoxide) Rb + O2 → RbO2 (superoxide) Cs + O2 → CsO2 (superoxide) The oxides of Group 1A Direct reaction of the alkali metals with O2 gives : Li ‐> oxide, peroxide (trace) Na ‐> peroxide , oxide (trace) K,Rb,Cs ‐> superoxide Diagonal Relationships: The Special Case of Lithium In some of its properties, lithium and its compounds resemble magnesium and its compounds. -

Where Is Water on the Periodic Table
Where Is Water On The Periodic Table Grum Jess regrown some tirls and risk his creepies so ambrosially! Roderigo is edaphic: she libel ahold and budges her barm. Corrugate and percipient Ford never digitized lickerishly when Janos literalising his ultrafilter. Unsubscribe from rivers from three particles in marah was it is where water on the periodic table The periodic tables is where compounds is a question yourself. Periodic table organized his element does not the water where is on oxygen and silicon does the universe and the sight of concentration and. In the marine environment. The table is where it is generally halogens are. An appreciation of water resources, data for gym, make tungsten gets very sophisticated pieces of oxo anions. Tcp connection time is table is on the water periodic. In the 9th century BC a Spartan lawgiver invented a drinking cup that could go mud stick missing its turn Later on the posture of medicine Hippocrates developed a device called the Hippocrates Sleeve a cloth bag weight was used to strain boiled rain water eliminating hoarseness and even smell. His law that is an apartment building large version of a ratio of where water was given element in open a pond would accumulate at an outer shells need. On their properties? Free 2-day shipping Buy Chemistry Elements Pet Mat the Food make Water Periodic Table of Elements in Green Shades Education Themed Non-Slip Rubber. The wall of the cell is the plasma membrane that controls the rate and type of ions and molecules passing into and out of the cell. -

Actinide Ground-State Properties-Theoretical Predictions
Actinide Ground-State Properties Theoretical predictions John M. Wills and Olle Eriksson electron-electron correlations—the electronic energy of the ground state of or nearly fifty years, the actinides interactions among the 5f electrons and solids, molecules, and atoms as a func- defied the efforts of solid-state between them and other electrons—are tional of electron density. The DFT Ftheorists to understand their expected to affect the bonding. prescription has had such a profound properties. These metals are among Low-symmetry crystal structures, impact on basic research in both the most complex of the long-lived relativistic effects, and electron- chemistry and solid-state physics that elements, and in the solid state, they electron correlations are very difficult Walter Kohn, its main inventor, was display some of the most unusual to treat in traditional electronic- one of the recipients of the 1998 behaviors of any series in the periodic structure calculations of metals and, Nobel Prize in Chemistry. table. Very low melting temperatures, until the last decade, were outside the In general, it is not possible to apply large anisotropic thermal-expansion realm of computational ability. And DFT without some approximation. coefficients, very low symmetry crystal yet, it is essential to treat these effects But many man-years of intense research structures, many solid-to-solid phase properly in order to understand the have yielded reliable approximate transitions—the list is daunting. Where physics of the actinides. Electron- expressions for the total energy in does one begin to put together an electron correlations are important in which all terms, except for a single- understanding of these elements? determining the degree to which 5f particle kinetic-energy term, can be In the last 10 years, together with electrons are localized at lattice sites. -

Basic Keyword List
NOTICE TO AUTHORS 2008 Basic Keyword list A Antibodies Bismuth Chalcogens Ab initio calculations Antifungal agents Block copolymers Chaperone proteins Absorption Antigens Bond energy Charge carrier injection Acidity Antimony Bond theory Charge transfer Actinides Antisense agents Boranes Chelates Acylation Antitumor agents Borates Chemical vapor deposition Addition to alkenes Antiviral agents Boron Chemical vapor transport Addition to carbonyl com- Aqueous-phase catalysis Bridging ligands Chemisorption pounds Arene ligands Bromine Chemoenzymatic synthesis Adsorption Arenes Brønsted acids Chemoselectivity Aerobic oxidation Argon Chiral auxiliaries Aggregation Aromatic substitution C Chiral pool Agostic interactions Aromaticity C-C activation Chiral resolution Alanes Arsenic C-C bond formation Chirality Alcohols Arylation C-C coupling Chlorine Aldehydes Aryl halides C-Cl bond activation Chromates Aldol reaction Arynes C-Glycosides Chromium Alkali metals As ligands C-H activation Chromophores Alkaline earth metals Asymmetric amplification C1 building blocks Circular dichroism Alkaloids Asymmetric catalysis Cadmium Clathrates Alkanes Asymmetric synthesis Cage compounds Clays Alkene ligands Atmospheric chemistry Calcium Cleavage reactions Alkenes Atom economy Calixarenes Cluster compounds Alkylation Atropisomerism Calorimetry Cobalamines Alkyne ligands Aurophilicity Carbanions Cobalt Alkynes Autocatalysis Carbene homologues Cofactors Alkynylation Automerization Carbene ligands Colloids Allenes Autoxidation Carbenes Combinatorial chemistry Allosterism -

Chapter 7 Electron Configuration and the Periodic Table
Chapter 7 Electron Configuration and the Periodic Table Copyright McGraw-Hill 2009 1 7.1 Development of the Periodic Table • 1864 - John Newlands - Law of Octaves- every 8th element had similar properties when arranged by atomic masses (not true past Ca) • 1869 - Dmitri Mendeleev & Lothar Meyer - independently proposed idea of periodicity (recurrence of properties) Copyright McGraw-Hill 2009 2 • Mendeleev – Grouped elements (66) according to properties – Predicted properties for elements not yet discovered – Though a good model, Mendeleev could not explain inconsistencies, for instance, all elements were not in order according to atomic mass Copyright McGraw-Hill 2009 3 • 1913 - Henry Moseley explained the discrepancy – Discovered correlation between number of protons (atomic number) and frequency of X rays generated – Today, elements are arranged in order of increasing atomic number Copyright McGraw-Hill 2009 4 Periodic Table by Dates of Discovery Copyright McGraw-Hill 2009 5 Essential Elements in the Human Body Copyright McGraw-Hill 2009 6 The Modern Periodic Table Copyright McGraw-Hill 2009 7 7.2 The Modern Periodic Table • Classification of Elements – Main group elements - “representative elements” Group 1A- 7A – Noble gases - Group 8A all have ns2np6 configuration(exception-He) – Transition elements - 1B, 3B - 8B “d- block” – Lanthanides/actinides - “f-block” Copyright McGraw-Hill 2009 8 Periodic Table Colored Coded By Main Classifications Copyright McGraw-Hill 2009 9 Copyright McGraw-Hill 2009 10 • Predicting properties – Valence -

Chemistry – Inorganic Chemistry
Answer on Question #53306 – Chemistry – Inorganic Chemistry Question What is oxidation state? How can find out the oxidation state of particular element? Explain its trend in the group and period, give reasons Answer The oxidation state is an indicator of the degree of oxidation (loss of electrons) of an atom in a chemical compound. The oxidation state, which may be positive, negative or equal to zero, is the hypothetical charge that an atom would have if all bonds to atoms of different elements were completely ionic, with no covalent component. To find out the oxidation state of particular element one should use some simple rules: 1. The oxidation state of an element in a simple substance (for example, He or Cl2, or Fe, or C, or whatever containing one type of atoms) is equal to zero. 2. The sum of the oxidation states of all the atoms or ions in a neutral compound is zero. 3. The sum of the oxidation states of all the atoms in an ion is equal to the charge on the ion. 4. The more electronegative element in a substance is given a negative oxidation state. The less electronegative one is given a positive oxidation state. 5. Some elements almost always have the same oxidation states in their compounds: Element Oxidation state Group 1 metals (Li, Na, K, Rb, Cs, Fr) always +1 Group 2 metals (Be, Mg, Ca, Sr, Ba, Ra) always +2 Fluorine (F) always -1 Oxygen (O) usually -2 (except in peroxides (-1) and F2O (+2)) Hydrogen (H) usually +1 (except in metal hydrides (-1)) Having known the oxidation states of these elements in the compound and having known the rule 3, the oxidation state of particular element can be found. -

Of the Periodic Table
of the Periodic Table teacher notes Give your students a visual introduction to the families of the periodic table! This product includes eight mini- posters, one for each of the element families on the main group of the periodic table: Alkali Metals, Alkaline Earth Metals, Boron/Aluminum Group (Icosagens), Carbon Group (Crystallogens), Nitrogen Group (Pnictogens), Oxygen Group (Chalcogens), Halogens, and Noble Gases. The mini-posters give overview information about the family as well as a visual of where on the periodic table the family is located and a diagram of an atom of that family highlighting the number of valence electrons. Also included is the student packet, which is broken into the eight families and asks for specific information that students will find on the mini-posters. The students are also directed to color each family with a specific color on the blank graphic organizer at the end of their packet and they go to the fantastic interactive table at www.periodictable.com to learn even more about the elements in each family. Furthermore, there is a section for students to conduct their own research on the element of hydrogen, which does not belong to a family. When I use this activity, I print two of each mini-poster in color (pages 8 through 15 of this file), laminate them, and lay them on a big table. I have students work in partners to read about each family, one at a time, and complete that section of the student packet (pages 16 through 21 of this file). When they finish, they bring the mini-poster back to the table for another group to use.