2021.01.27.21250591V1.Full.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

• Cytosis: O Neutrophilia: Defined As an Increase in the Neutrophilic Count in the Peripheral Blood Above Reference Range for Age
HENATOLYMPHOID SYSTEM THIRD YEAR MEDICAL STUDENTS-UNIVERSITY OF JORDAN AHMAD T. MANSOUR, MD NONNEOPLASTIC DISEASES OF THE WHITE BLOOD CELLS • There are five major types of WBCs in the blood: neutrophils, lymphocytes, eosinophils, basophils and monocytes. • The normal function of the white blood cells depends on a tight regulation of their count and their function. Therefore, disease develops if there is a derangement of the cells count or function, it takes one of the following forms: o Cytosis: increase in the number of circulating cells above reference range. (Note: leukocytosis means an increase in the WBC count, neutrophilia means increase in the neutrophilic count, lymphocytosis means increase in the lymphocytic count, monocytosis means increase in the monocytic count, basophilia means increase in the basophilic count and eosinophilia means in crease in the eosinophilic count). o Cytopenia: decrease in the number of circulating cells below reference range. (Note: neutropenia means decreased neutrophils, lymphocytopenia, or simply lymphopenia, means decrease in lymphocytes, monocytopenia means decrease in monocytes, eosinopenia means decrease in eosinophils, and basopenia means decrease in basophils). o Abnormal or absent function • Cytosis: o Neutrophilia: defined as an increase in the neutrophilic count in the peripheral blood above reference range for age. o Causes: bacterial infection is the most common and most important etiology. Tissue necrosis in cases of burns or trauma and medications such as epinephrine and corticosteroids are also additional causes for neutrophilia. § Some physiologic conditions can lead to neutrophilia such as stress, smoking and pregnancy. o Pathophysiology: neutrophils are present in the blood in two populations: circulating and marginal (meaning neutrophils stuck to the vessel wall). -
Complete Blood Count in Primary Care
Complete Blood Count in Primary Care bpac nz better medicine Editorial Team bpacnz Tony Fraser 10 George Street Professor Murray Tilyard PO Box 6032, Dunedin Clinical Advisory Group phone 03 477 5418 Dr Dave Colquhoun Michele Cray free fax 0800 bpac nz Dr Rosemary Ikram www.bpac.org.nz Dr Peter Jensen Dr Cam Kyle Dr Chris Leathart Dr Lynn McBain Associate Professor Jim Reid Dr David Reith Professor Murray Tilyard Programme Development Team Noni Allison Rachael Clarke Rebecca Didham Terry Ehau Peter Ellison Dr Malcolm Kendall-Smith Dr Anne Marie Tangney Dr Trevor Walker Dr Sharyn Willis Dave Woods Report Development Team Justine Broadley Todd Gillies Lana Johnson Web Gordon Smith Design Michael Crawford Management and Administration Kaye Baldwin Tony Fraser Kyla Letman Professor Murray Tilyard Distribution Zane Lindon Lyn Thomlinson Colleen Witchall All information is intended for use by competent health care professionals and should be utilised in conjunction with © May 2008 pertinent clinical data. Contents Key points/purpose 2 Introduction 2 Background ▪ Haematopoiesis - Cell development 3 ▪ Limitations of reference ranges for the CBC 4 ▪ Borderline abnormal results must be interpreted in clinical context 4 ▪ History and clinical examination 4 White Cells ▪ Neutrophils 5 ▪ Lymphocytes 9 ▪ Monocytes 11 ▪ Basophils 12 ▪ Eosinophils 12 ▪ Platelets 13 Haemoglobin and red cell indices ▪ Low haemoglobin 15 ▪ Microcytic anaemia 15 ▪ Normocytic anaemia 16 ▪ Macrocytic anaemia 17 ▪ High haemoglobin 17 ▪ Other red cell indices 18 Summary Table 19 Glossary 20 This resource is a consensus document, developed with haematology and general practice input. We would like to thank: Dr Liam Fernyhough, Haematologist, Canterbury Health Laboratories Dr Chris Leathart, GP, Christchurch Dr Edward Theakston, Haematologist, Diagnostic Medlab Ltd We would like to acknowledge their advice, expertise and valuable feedback on this document. -

Anemic Syndrome and White Blood Cells Disorders
27. 11. 2020 Anemic syndrome and white blood cells disorders Kristína Repová, M.D., PhD. [email protected] Institute of Pathophysiology, Faculty of Medicine, Bratislava Prepared exclusively for the purposes of distance education at the Faculty of Medicine, Comenius University in Bratislava in 2020/21 Hematopoeisis • Hematopoietic organs: • Bone marrow: • forming of erythrocytes, granulocytes, monocytes, thrombocytes, partially lymphocytes • Thymus: • forming of T-lymphocytes • Lymphatic nodes, tonsils, spleen: • forming of B-lymphocytes lymphoid multipotent stem cell pluripotent progenitor cell precursor cell stem cell myleoid multipotent stem cell 1 27. 11. 2020 Hematopoeisis 3 Pluripotent hematopoietic stem cell (self-renewal) Myeloid multipotent Lymphoid multipotent stem cell stem cell Megacaryocyte and Granulocyte and T-cell and NK B-cell erythroid progenitor Macrophage progenitor cell progenitor progenitor Megacaryocyte Erythrocyte Granulocyte Monocyte progenitor progenitor progenitor progenitor (CFU-Meg) (CFU-E) (CFU-G) (CFU-M) Myeloblast NK-cell Proerythroblast Monoblast Lymphoblast Lymphoblast Promyelocyte Megacaryoblast Erythroblast Myelocyte Promonocyte Prolymphocyte Prolymphocyte Megacaryocyte Reticulocyte Metamyelocyte Monocyte T-cell B-cell Thrombocyte Erythrocyte Band cell Basophil Eosinophil Macrophage Dendritic cell Neutrophil 2 27. 11. 2020 I. Disorders of red blood cells II. Disorders of white blood cells III. Myeloproliferative and lymphoproliferative disorders I. Disorders of red blood cells 1. Anemia 2. -

Pub 100-04 Medicare Claims Processing
Department of Health & CMS Manual System Human Services (DHHS) Pub 100-04 Medicare Claims Centers for Medicare & Processing Medicaid Services (CMS) Transmittal 990 Date: JUNE 23, 2006 Change Request 5142 Subject: Medicare Contractor Annual Update of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) I. SUMMARY OF CHANGES: This instruction is CMS' annual reminder to the contractors of the ICD-9-CM update that is effective for the dates of service on and after October 1, 2006, as well as discharges on or after October 1, 2006 for institutional providers. New / Revised Material Effective Date: October 1, 2006 Implementation Date: October 2, 2006 Disclaimer for manual changes only: The revision date and transmittal number apply only to red italicized material. Any other material was previously published and remains unchanged. However, if this revision contains a table of contents, you will receive the new/revised information only, and not the entire table of contents. II. CHANGES IN MANUAL INSTRUCTIONS: (N/A if manual is not updated) R=REVISED, N=NEW, D=DELETED-Only One Per Row. R/N/D Chapter / Section / Subsection / Title N/A III. FUNDING: No additional funding will be provided by CMS; Contractor activities are to be carried out within their FY 2006 operating budgets. IV. ATTACHMENTS: Recurring Update Notification *Unless otherwise specified, the effective date is the date of service. Attachment – Recurring Update Notification Pub. 100-04 Transmittal: 990 Date: June 23, 2006 Change Request 5142 SUBJECT: Medicare Contractor Annual Update of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) I. -

Evaluation of White Blood Cells Picture (Leukocytes) I-General the Term Leukocyte Include All White Blood Cells and Their Precursors
Dr.Iman Daham , BSci., MSc., PhD. Assist. Prof., Department of Internal and Preventive Medicine College of Veterinary Medicine, University of Mosul, Mosul, Iraq https://orcid.org/0000-0002-0947-7169 https://www.researchgate.net/profile/Iman Daham Clinical Pathology | Part I | 4th year 2019 Evaluation of White Blood Cells Picture (Leukocytes) I-General The term leukocyte include all white blood cells and their precursors. These cells use blood stream as a means of transport from their site of origin to the site in tissues where they are required . The circulating numbers therefore reflect the balance between supply and demand , and usually rang between about 5-14 ×10/L depending to some extent on species. The leukocytes can be divided in two basic categories: 1-The granulocytes which include Neutrophils , Eosinophil’s and Basophils. 2-Agranulocytes which include Lymphocytes and Monocytes. II-Alteration of the Leukocyte picture 1-Leukocytosis A-Physiological leukocytosis : a-age of animal: Total leukocyte count are higher in the young than in the adult of Dog and Cattle. Lowered leukocyte count in young animals than in adults appeared in Swine. No significant difference in total leukocyte count of young animal and adults in Horse and Sheep. b-Digestion is accompanied with leukocytosis as in Dog (one hour after digestion that reached its maximum about 3-4 hours and then declines) Horses have a weak digestion leukocytosis. c-Epinephrine injection. d-Pregnancy in cattle induces Leukocytosis with Neutrophilia especially two weeks before parturition. B-Pathological Leukocytosis: a-Acute infection by pyogenic bacteria as 1-Staphilococcus 2-Streptococcus. b-Rabies virus infection induces mild Leukocytosis. -

Hematological Profile of Normal Pregnant Women
Blood of & al L n y Mutua et al., J Blood Lymph 2018, 8:2 r m u p o h J Journal of Blood & Lymph DOI: 10.4172/2165-7831.1000220 ISSN: 2165-7831 Review Article Open Access Hematological Profile of Normal Pregnant Women David Nzioka Mutua1,2*, Eliud Nyaga Mwaniki Njagi1 and George Owino Orinda1 1Department of Biochemistry and Biotechnology, School of Pure and Applied Sciences, Kenyatta University, Nairobi, Kenya 2Department of Medical Biochemistry, School of Medicine and Health Sciences, Kenya Methodist University, Meru, Kenya Abstract With the advent of many interventions to improve maternal and child health, pregnant women have become the focus of many health programs. However, few data exist regarding this important population. Although pregnancy- induced changes occur in hematological values, very few laboratories provide specific reference ranges for pregnant women. Most laboratory information systems report reference values based on samples obtained from non–pregnant women which may not be useful for clinical decisions during pregnancy. Thus, there is an increased risk of overlooking important physiologic alterations resulting from pathological conditions and of misinterpreting normal changes as pathological events. It is therefore important to understand pregnancy-induced hematological changes for correct clinical evaluation of pregnant women. In this review, we discuss complete blood count and the associated pregnancy-induced hematological changes. We also highlight the dynamic changes of these parameters per trimester and show how they differ between populations. Keywords: Pregnancy; Complete blood count; Hematological Hematocrit (Hct), Hemoglobin (Hb or Hgb), Mean Corpuscular changes; Trimester Volume (MCV), Mean Corpuscular Hemoglobin (MCH), and Mean Corpuscular Hemoglobin Concentration (MCHC) [5,26]. -

Wight Blood Pathology
WHITE BLOOD PATHOLOGY Alteration of leukocytes function, leukemia Normal distribution of white blood cells Total white cells count 4,0 – 11,0 x 10 9/L CELL ABSOLUTE PER sent % NUMBER x109 /L Neutrophils 2,5 – 7,5 58 - 72 BAND cells 0,04 – 0,4 1 - 6 Lymphocytes 1,5 – 4,0 19 -37 Monocytes 0,2 – 0,8 3 - 11 Eosinophils 0,04 – 0,4 1 - 6 Basophiles 0,01 – 0,1 0,5 - 1 Calculation of absolute or relative leukocytosis Example: Blood examination: Leukocytes – 1,5 x 109 /l segmented neutrophils – 15% Lymphocytes - 70% Estimate the absolute amount of neutrophils and lymphocytes Total amount of leukocytes 1,5 x 109 - 100% neutrophils X - 15% X= ( 1,5 x 109 x 15 ) : 100 = 0,225 x 109 ( 2,5 – 7,5 x109 ) Absolute neutropenia Total amount of leukocytes 1,5 x 10 9 - 100% lymphocytes X - 70% X = ( 1,5 x 109 x 70 ): 100 = 1,05 x 10 9 ( 1,5 – 4 x 10 9 ) Relative lymphocytosis Leukocytosis - ↑amount of L > 9 x 10 9 /L NEUTROPHILIA ← Causes: - infection, - APR, - tissue injury - hemorrhage, - neoplasm, - metabolic disorders, - stress states, - inflammation, - severe colic, - glucocorticoid administration. MONOCYTOSIS ← -chronic infection: tuberculosis, lepra, siphilis, malaria, rikketsiosis, endocarditis - infection mononucleosis, - vasculitis, - collagen disease EOSINOPHYLIA ← - allergy, - atopic diseases, - neoplasms, - chronic paracitic invasion, - dermatologic disorders LYMPHOCYTOSIS ← - acute viral infection, - hepatitis, - typhoid, - thyrotoxicosis, - adrenal insufficiency - infectious mononucleosis LYMPHOCYTOSIS NON – MELIGNANT CAUSES OF LYMPHOSITOSIS -

The Full Blood Count Blood Test for Colorectal Cancer Detection: a Systematic Review, Meta-Analysis, and Critical Appraisal
Review The Full Blood Count Blood Test for Colorectal Cancer Detection: A Systematic Review, Meta-Analysis, and Critical Appraisal Pradeep S. Virdee, Ioana Marian, Anita Mansouri, Leena Elhussein, Shona Kirtley, Tim Holt and Jacqueline Birks 1. Description of the full blood count Table S1. Description of each component of the full blood count blood test Full blood count Conventional Description/purpose component units The number of red blood cells in the blood. 1012 per litre Lower levels can be associated with blood less. Red blood cell count 1 (1012/L) Each red blood cell contains levels of haemoglobin. The number of white blood cells in the blood. White blood cell count 1 109 per litre (109/L) They are of the immune system and involved in protecting the body against disease. Grams per Carries oxygen around the body and is found in Haemoglobin 1 decilitre (g/dL) red blood cells Haematocrit/packed cell Percentage, i.e. as Proportion of blood that is occupied by the red volume 2 litres per litre (%) cells. A possible alternative for detecting anaemia. Average size of red blood cells. Calculated as Mean corpuscular Femtolitre (f/L) haematocrit (%) divided by the number of red volume 2 blood cells (1012/L) Average of amount of haemoglobin that is in each Mean corpuscular Pictograms (pg) red blood cell. Calculated as haemoglobin (g/dL) haemoglobin 2 divided by the red blood cell count (1012/L) Average of amount of haemoglobin in individual Mean corpuscular Grams per cells based on the volume of red blood cells. haemoglobin decilitre (g/dL) Calculated as haemoglobin (g/dL) divided by the concentration 2 haematocrit (%) Red cell distribution A coefficient, as A measure of the amount of variation in the size width 2 opposed to count of red cells; the higher, the more variation. -
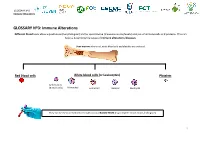
GLOSSARY Nº3: Immune Alterations
GLOSSARY Nº3 Immune Alterations GLOSSARY Nº3: Immune Alterations Different blood tests allow a qualitative (morphological) and/or quantitative (measure counts/levels) analysis of immune cells and proteins. This can help us determine the cause of immune alterations/diseases. Bone marrow: where red, white blood cells and platelets are produced Red Blood cells White blood cells (or Leukocytes) Platelets Lymphocytes (B and T cells) Monocytes Eosinophil Basophil Neutrophil They can be measured/monitored with various BLOOD TESTS (e.g complete blood count, leukogram) 1 GLOSSARY Nº3 Immune Alterations Types of Immune What is it? What does it mean? Alterations What does it cause? Basophilia High levels of basophils (a type of white blood cell important in allergies) It can be indicative of an allergy (usually associated with itching), infection, autoimmune diseases or cancer Basopenia Low levels of basophils (a type of white blood cell important in allergies) It is often associated with allergies (e.g urticaria) and autoimmune diseases. Low levels of some immunoglobulins (antibodies). There are 5 different classes of antibodies: Increased susceptibility to develop infections Dysgammaglobulinemia IgA, IgG, IgM, IgE, IgD and, in this case, one or more (but, NOT ALL) are decreased. with potential serious complications. Increased susceptibility to develop infections Hypogammaglobulinema Low levels of ALL immunoglobulins (antibodies). with potential serious complications. It could indicate a parasitic infection, allergy, Eosinophilia High levels of eosinophils (a type of white blood cell important in fighting different infections). autoimmune disease or cancer. It is often associated with acute inflammation Eosinopenia Low levels of eosinophils (a type of white blood cell important in fighting different infections). -

Distribution of Hematological Parameters Counts for Children with Leukemia in Children’S Cancer Units at Al-Kuwait Hospital, Sana’A City: a Cross-Sectional Study
ISSN: 2688-8203 DOI: 10.33552/ACRCI.2021.03.000559 Advances in Cancer Research & Clinical Imaging Research Article Copyright © All rights are reserved by Hassan A Al-Shamahy Distribution of Hematological Parameters Counts for Children with Leukemia in Children’s Cancer Units at Al-Kuwait Hospital, Sana’a City: A Cross-Sectional Study Lutfi AS Al-Maktari1, Mohamed AK Al-Nuzaili1, Hassan A Al-Shamahy2*, Abdulrahman A Al-Hadi3, Abdulrahman A Ishak3 and Saleh A Bamashmoos1 1Department of Hematology, Faculty of Medicine and Health Sciences, Sana’a University, Republic of Yemen 2Department of Medical Microbiology and Clinical Immunology Department, Faculty of Medicine and Health Sciences, Sana’a University, Republic of Yemen 3Department of Pediatric, Faculty of Medicine and Health Sciences, Sana’a University, Republic of Yemen *Corresponding author: Hassan A Al-Shamahy, Faculty of Medicine and Heath Received Date: June 14, 2021 Sciences, Sana’a University, P.O. Box 775 Sana’a, Yemen. Published Date: June 30, 2021 Abstract Introduction and aims: Leukemia is a heterogeneous group of hematological disorders that is made up of several diverse and biologically distinct subgroups. Leukemia is the eleventh and tenth most common cause of cancer morbidity and mortality worldwide, respectively. There are parameters counts for children with leukemia. insufficient data on the hematological parameters counts of leukemia in Yemen. This cross-sectional study aims to determine the hematological Materials and methods: A cross-sectional study was conducted on children with leukemia who were treated selectively in the pediatric leukemia units of Kuwait University Hospital in Sana’a. Group diagnostics and histopathological diagnoses were formed in line with the French, American 2018. -

Chapter One Non-Neoplastic Disorders Of
PART I NON-NEOPLASTIC HEMATOLOGY COPYRIGHTED MATERIAL CHAPTER ONE NON-NEOPLASTIC DISORDERS OF WHITE BLOOD CELLS Rebecca A. Levy, Vandita P. Johari, and Liron Pantanowitz OVERVIEW OF WBC PRODUCTION Leukocytes AND FUNCTION Hematopoeisis Hematopoiesis occurs in different parts of the Frequently the first test that suggests an imbalance or body, depending on the age of the embryo, child, or disturbance in hematopoiesis is the complete blood adult. Initially blood cell formation of the embryo count (CBC). The CBC is a simple blood test that occurs within the yolk sac, in blood cell aggregates is ordered frequently. It may pick up incidental called blood islands. As development progresses, abnormalities or may yield a diagnosis of suspected the hematopoiesis location changes, and the spleen abnormalities. The CBC is a count of multiple blood and liver become the primary sites. As bone marrow components and qualities, and can include a differ- develops, it usurps the task of blood cell formation ential of the white blood cells (WBCs). The CBC can and becomes the site for trilineage hematopoiesis. suggest infection, inflammatory processes, and malig- The bone marrow contains pluripotent stem cells, nant processes. Typically a peripheral blood smear which can develop into any of the blood cells, includ- and rarely a buffy coat (concentrated white blood ing granulocytes, monocytes, and lymphocytes, in cells) are analyzed to help determine a diagnosis response to specific stimulating factors (Andrews, (Efrati, 1960). A differential WBC assigns leukocytes 1994). Several white blood cells (leukocytes) are to their specific categories as a percentage or absolute depicted in Figure 1.1. -

A) Increase in the Number of Leukocytes in the Bone Marrow + B
Leukocytosis 1.What a leukocytosis? a) Increase in the number of leukocytes in the bone marrow + b) Increasing the number of leucocytes per unit volume of blood (usually over 9.0 gig / liter) c) Increasing the number of leukocytes in tissues. 2. What is the number of white blood cells indicates a leukocytosis? a) 2.0 gig / liter; + b) 15.0 gig / liter; c) 9.0 gig / liter. 3. What mechanisms play a major role in the emergence of leukocytosis? + a) Stimulation leukopoiesis + b) Accelerated exit of leukocytes in peripheral blood c) Increased capillary pressure d) Decrease capillary pressure 4. Index of nuclear shift - is: + a) the ratio of all non-segmentonuclear neutrophils in percent to the percentage of segmentonuclear cells b) the ratio of all segmentonuclear to the sum of all non-segmentonuclear neutrophils. 5. What is the index of the nuclear shift of neutrophils in normal? + a) 0.06 - 0.08; b) 0.9 - 1.0; c) 1.2 - 2.0. 6. Neutrophilic leukocytosis observed in: a) Allergic reactions + b) pyoseptic processes c) helminthic invasion + d) Traumatic brain injury + e) Myocardial Infarction 7. What leukocytosis usually occurs after acute blood loss? + a) neutrophilic; b) eosinophilic; c) lymphocytic. 8. Physiological leukocytosis observed in: a) Myocardial Infarction b) Pneumonia c) Bone fractures + d) Neonatal e) Acute posthemorrhagic anemia 9. General mechanism is pathological leukocytosis a) The redistribution of blood in the vessels + b) Stimulation leukopoiesis c) The emigration of leukocytes d) diapedesis of leukocytes e) phagocytosis of leukocytes 10. Eosinophilia characterized by: a) acute purulent process + b) for asthma c) Sepsis d) For infectious mononucleosis e) For Measles 11.