Classical Biological Control of Nodding and Plumeless Thistles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rule 40-12-4-.01 Limitations on Noxious Weed Seeds(MARKED)
Rule 40-12-4-.01. Limitations on Noxious Weed Seeds It is unlawful to sell, offer for sale, or expose for sale, any agricultural or vegetable seed for planting purposes in this State if the noxious weed seeds per pound of pure seed is in excess of the following limitations: (a) Prohibited Noxious Weed Seeds. Name Limitations 1. Balloonvine (Cardiospermum halicacabum)………………..…………….....Prohibited 2. Bindweed, Field (Convolvulus arvensis) ……….………………...…….…..Prohibited 3. Bindweed, Hedge (Calystegia sepium) ………….………………...………..Prohibited 4. Cocklebur (Xanthium spp.) …………………….…………………...…..…..Prohibited 5. Crotalaria (Crotalaria spp.) …………………….…………………...…….....Prohibited 6. Morningglory, Giant or Moonflower (Ipomoea turbinata) ………………………………………………....…..Prohibited 7. Nutsedge, Purple (Cyperus rotundus) ………………...………………...…..Prohibited 8. Nutsedge, Yellow (Cyperus esculentus).. ……….….………………..……..Prohibited 9. Tropical Soda Apple (Solanum viarum) …………………………...…...…..Prohibited 10. Tussock, Serrated (Nassella trichotoma) ………………………………..…..Prohibited Genus Species Common Name Limitations Acalypha ostryifolia Hophornbeam Copperleaf Prohibited Acalypha virginica Three-seeded mercury Prohibited Calystegia Spp. Hedge Bindweed Prohibited Cardiospermum halicacabum Balloonvine Prohibited Convolvulus arvensis Bindweed, Field Prohibited Convolvulus Equitans Bindweed, hoary Prohibited Conyza canadensis Horseweed (Marestail) Prohibited Crotalaria Spp. Crotalaria Prohibited Cyperus esculentus Nutsedge, Yellow Prohibited Cyperus rotundus Nutsedge, Purple Prohibited -

Thistles of Colorado
Thistles of Colorado About This Guide Identification and Management Guide Many individuals, organizations and agencies from throughout the state (acknowledgements on inside back cover) contributed ideas, content, photos, plant descriptions, management information and printing support toward the completion of this guide. Mountain thistle (Cirsium scopulorum) growing above timberline Casey Cisneros, Tim D’Amato and the Larimer County Department of Natural Resources Weed District collected, compiled and edited information, content and photos for this guide. Produced by the We welcome your comments, corrections, suggestions, and high Larimer County quality photos. If you would like to contribute to future editions, please contact the Larimer County Weed District at 970-498- Weed District 5769 or email [email protected] or [email protected]. Front cover photo of Cirsium eatonii var. hesperium by Janis Huggins Partners in Land Stewardship 2nd Edition 1 2 Table of Contents Introduction 4 Introduction Native Thistles (Pages 6-20) Barneyby’s Thistle (Cirsium barnebyi) 6 Cainville Thistle (Cirsium clacareum) 6 Native thistles are dispersed broadly Eaton’s Thistle (Cirsium eatonii) 8 across many Colorado ecosystems. Individual species occupy niches from Elk or Meadow Thistle (Cirsium scariosum) 8 3,500 feet to above timberline. These Flodman’s Thistle (Cirsium flodmanii) 10 plants are valuable to pollinators, seed Fringed or Fish Lake Thistle (Cirsium 10 feeders, browsing wildlife and to the centaureae or C. clavatum var. beauty and diversity of our native plant americanum) communities. Some non-native species Mountain Thistle (Cirsium scopulorum) 12 have become an invasive threat to New Mexico Thistle (Cirsium 12 agriculture and natural areas. For this reason, native and non-native thistles neomexicanum) alike are often pulled, mowed, clipped or Ousterhout’s or Aspen Thistle (Cirsium 14 sprayed indiscriminately. -

Italian Thistle (Carduus Pycnocephalus)
Thistles: Identification and Management Rebecca Ozeran 1 May 2018 Common thistles in the San Joaquin Valley Carduus Centaurea Cirsium Silybum Onopordum Italian thistle Yellow starthistle Bull thistle (Blessed) milkthistle Scotch thistle Tocalote Canada thistle (Malta starthistle) All of these species are found at least one of Fresno, Kern, Kings, Madera, or Tulare Counties Identification • Many species start as a basal rosette in fall • Mature plants can have dense & bushy or tall & stemmy appearance • Purple/pink or yellow-flowered Identification • Why does thistle species matter? • Varying levels of risk to animals • Varying competition with forage • Varying susceptibility to control options Identification – 1. Italian thistle • Carduus pycnocephalus • narrow, spiky flower heads • winged, spiny stems branching above the base • found in Fresno, Kern, Madera, Tulare Identification – 2. Centaurea thistles • YELLOW STARTHISTLE (C. solstitialis) • long, yellow/white spines on phyllaries • can get a bushy structure • found in Fresno, Kern, Madera, Tulare • TOCALOTE (MALTA STARTHISTLE, C. melitensis) • stouter flower heads and shorter, redder spines on phyllaries • found in all 5 counties Identification – 3. Cirsium thistles • Canada thistle (C. arvense) • smooth stems, non-spiny flowerheads • flowers Jun-Oct • found in Fresno, Kern, Tulare • Bull thistle (C. vulgare) • large spiky looking flowerheads • lots of branching, dense plant • flowers Jun-Oct • found in all 5 counties Identification – 4. Blessed milk thistle • Silybum marianum • Distinct, -

Hymenoptera: Eulophidae) 321-356 ©Entomofauna Ansfelden/Austria; Download Unter
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Entomofauna Jahr/Year: 2007 Band/Volume: 0028 Autor(en)/Author(s): Yefremova Zoya A., Ebrahimi Ebrahim, Yegorenkova Ekaterina Artikel/Article: The Subfamilies Eulophinae, Entedoninae and Tetrastichinae in Iran, with description of new species (Hymenoptera: Eulophidae) 321-356 ©Entomofauna Ansfelden/Austria; download unter www.biologiezentrum.at Entomofauna ZEITSCHRIFT FÜR ENTOMOLOGIE Band 28, Heft 25: 321-356 ISSN 0250-4413 Ansfelden, 30. November 2007 The Subfamilies Eulophinae, Entedoninae and Tetrastichinae in Iran, with description of new species (Hymenoptera: Eulophidae) Zoya YEFREMOVA, Ebrahim EBRAHIMI & Ekaterina YEGORENKOVA Abstract This paper reflects the current degree of research of Eulophidae and their hosts in Iran. A list of the species from Iran belonging to the subfamilies Eulophinae, Entedoninae and Tetrastichinae is presented. In the present work 47 species from 22 genera are recorded from Iran. Two species (Cirrospilus scapus sp. nov. and Aprostocetus persicus sp. nov.) are described as new. A list of 45 host-parasitoid associations in Iran and keys to Iranian species of three genera (Cirrospilus, Diglyphus and Aprostocetus) are included. Zusammenfassung Dieser Artikel zeigt den derzeitigen Untersuchungsstand an eulophiden Wespen und ihrer Wirte im Iran. Eine Liste der für den Iran festgestellten Arten der Unterfamilien Eu- lophinae, Entedoninae und Tetrastichinae wird präsentiert. Mit vorliegender Arbeit werden 47 Arten in 22 Gattungen aus dem Iran nachgewiesen. Zwei neue Arten (Cirrospilus sca- pus sp. nov. und Aprostocetus persicus sp. nov.) werden beschrieben. Eine Liste von 45 Wirts- und Parasitoid-Beziehungen im Iran und ein Schlüssel für 3 Gattungen (Cirro- spilus, Diglyphus und Aprostocetus) sind in der Arbeit enthalten. -

The Curculionoidea of the Maltese Islands (Central Mediterranean) (Coleoptera)
BULLETIN OF THE ENTOMOLOGICAL SOCIETY OF MALTA (2010) Vol. 3 : 55-143 The Curculionoidea of the Maltese Islands (Central Mediterranean) (Coleoptera) David MIFSUD1 & Enzo COLONNELLI2 ABSTRACT. The Curculionoidea of the families Anthribidae, Rhynchitidae, Apionidae, Nanophyidae, Brachyceridae, Curculionidae, Erirhinidae, Raymondionymidae, Dryophthoridae and Scolytidae from the Maltese islands are reviewed. A total of 182 species are included, of which the following 51 species represent new records for this archipelago: Araecerus fasciculatus and Noxius curtirostris in Anthribidae; Protapion interjectum and Taeniapion rufulum in Apionidae; Corimalia centromaculata and C. tamarisci in Nanophyidae; Amaurorhinus bewickianus, A. sp. nr. paganettii, Brachypera fallax, B. lunata, B. zoilus, Ceutorhynchus leprieuri, Charagmus gressorius, Coniatus tamarisci, Coniocleonus pseudobliquus, Conorhynchus brevirostris, Cosmobaris alboseriata, C. scolopacea, Derelomus chamaeropis, Echinodera sp. nr. variegata, Hypera sp. nr. tenuirostris, Hypurus bertrandi, Larinus scolymi, Leptolepurus meridionalis, Limobius mixtus, Lixus brevirostris, L. punctiventris, L. vilis, Naupactus cervinus, Otiorhynchus armatus, O. liguricus, Rhamphus oxyacanthae, Rhinusa antirrhini, R. herbarum, R. moroderi, Sharpia rubida, Sibinia femoralis, Smicronyx albosquamosus, S. brevicornis, S. rufipennis, Stenocarus ruficornis, Styphloderes exsculptus, Trichosirocalus centrimacula, Tychius argentatus, T. bicolor, T. pauperculus and T. pusillus in Curculionidae; Sitophilus zeamais and -
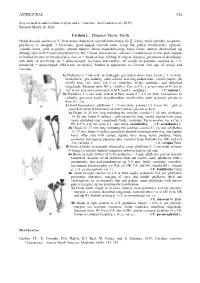
ASTERACEAE 552 Key Revised to Add Carduus Crispus and C. Cinereus
ASTERACEAE 552 Key revised to add Carduus crispus and C. cinereus. See Gaskin et al. (2019). Revised March 18, 2021. Carduus L. Plumeless Thistle; Thistle Heads discoid, solitary or 2–20 in dense clusters or corymbiform arrays; fls , fertile; invol cylindric to spheric, phyllaries ∞, unequal, 7–10-seriate, spiny-tipped, calyculi none; recep flat,⚥ pitted, bristly-scaly, epaleate; corollas white, pink to purple, slender-tubular, throat expanded-camp, lobes linear; anthers short-tailed, tip oblong; style with ± hairy ring below brs, brs ± linear, tips truncate; achenes ± compressed, ovoid, glab; pappus of barbed bristles or bristly-scales, free or ± fused at base (falling in ring or clusters), persistent or deciduous; ann, bien, or per herbs; sts ± spiny-winged; lvs basal and cauline, alt, sessile or petiolate, toothed to 1–2- pinnatifid, ± spiny-tipped. (Old name for thistle). Similar in appearance to Cirsium. Our spp. all weedy and noxious. 1a Phyllaries 2–7 mm wide at midlength, gen widest above base; heads 2–7 cm wide, hemispheric, gen nodding, often solitary and long-pedunculate; corolla purple; pls chiefly bien (occ ann), 0.4–2 m; wastelots, fields, roadsides, and disturbed rangelands; Eurasian intro; BC s, chiefly e Cas, to CA, e across most of N Am to Atl; in our area most common in w MT; musk t., nodding t. 1 C. nutans L. 1b Phyllaries 1–3 mm wide, widest at base; heads 0.7–2.5 cm wide, hemispheric to cylindric, gen erect, sessile or pedunculate; corolla white, pink, or purple; pls ann or bien, 0.1–2 m 1a 2a Invol hemispheric; phyllaries 1–1.5 mm wide; achenes 2.5–4 mm; lvs ± glab or sparsely to densely tomentose on lower surface; pls ann or bien 3a Heads 18–25 mm long including the corollas; corolla 13–20 mm; phyllaries 14–20 mm; lower lf surface ± glab except for long, curled, septate hairs along veins; disturbed sites, rangelands, fields, roadsides; Eurasian intro; occ e Cas, s BC to CA, e to Atl; spiny p. -

Terrestrial Insects: a Hidden Biodiversity Crisis? 1
Chapter 7—Terrestrial Insects: A Hidden Biodiversity Crisis? 1 Chapter 7 Terrestrial Insects: A Hidden Biodiversity Crisis? C.H. Dietrich Illinois Natural History Survey OBJECTIVES Like most other elements of the biota, the terrestrial insect fauna of Illinois has undergone drastic change since European colonization of the state. Although data are sparse or entirely lacking for most species, it is clear that many formerly abundant native species are now exceedingly rare while a few previously uncommon or undocumented species, both native and exotic, are now abundant. Much of this change may be attributable to fragmentation and loss of native habitats (e.g., deforestation, draining of wetlands, agricultural conversion and intensification, urbanization), although other factors such as invasion by exotic species (including plants, insects and pathogens), misuse of pesticides, and improper management of native ecosystems have probably also been involved. Data from Illinois and elsewhere in the north temperate zone provide evidence that at least some groups of terrestrial insects have undergone dramatic declines over the past several decades, suggesting that insects are no less vulnerable to anthropogenic environmental change than other groups of organisms Yet, insects continue to be under-represented on official lists of threatened or endangered species and conservation programs focus primarily on vertebrates and plants. This chapter summarizes available information on long-term changes in the terrestrial insect fauna of Illinois, reviews possible causes for these changes, highlights some urgent research needs, and provides recommendations for conservation and management of terrestrial insect communities. INTRODUCTION Insects are among the most important “little things that run the world” (1). -

Milk Thistle
Forest Health Technology Enterprise Team TECHNOLOGY TRANSFER Biological Control BIOLOGY AND BIOLOGICAL CONTROL OF EXOTIC T RU E T HISTL E S RACHEL WINSTON , RICH HANSEN , MA R K SCH W A R ZLÄNDE R , ER IC COO M BS , CA R OL BELL RANDALL , AND RODNEY LY M FHTET-2007-05 U.S. Department Forest September 2008 of Agriculture Service FHTET he Forest Health Technology Enterprise Team (FHTET) was created in 1995 Tby the Deputy Chief for State and Private Forestry, USDA, Forest Service, to develop and deliver technologies to protect and improve the health of American forests. This book was published by FHTET as part of the technology transfer series. http://www.fs.fed.us/foresthealth/technology/ On the cover: Italian thistle. Photo: ©Saint Mary’s College of California. The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, sex, religion, age, disability, political beliefs, sexual orientation, or marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at 202-720-2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, Room 326-W, Whitten Building, 1400 Independence Avenue, SW, Washington, D.C. 20250-9410 or call 202-720-5964 (voice and TDD). USDA is an equal opportunity provider and employer. The use of trade, firm, or corporation names in this publication is for information only and does not constitute an endorsement by the U.S. -

Invasive Alien Species: the Application of Classical Biological Control for the Management of Established Invasive Alien Species Causing Environmental Impacts
CBD Distr. GENERAL CBD/COP/14/INF/9 12 November 2018 ENGLISH ONLY CONFERENCE OF THE PARTIES TO THE CONVENTION ON BIOLOGICAL DIVERSITY Fourteenth meeting Item 26 of the provisional agenda* Sharm El-Sheikh, Egypt, 17-29 November 2018 INVASIVE ALIEN SPECIES: THE APPLICATION OF CLASSICAL BIOLOGICAL CONTROL FOR THE MANAGEMENT OF ESTABLISHED INVASIVE ALIEN SPECIES CAUSING ENVIRONMENTAL IMPACTS Note by the Executive Secretary BACKGROUND 1. The Executive Secretary is circulating herewith, for the information of participants in the fourteenth meeting of the Conference of the Parties, a document on the application of classical biological control for the management of established invasive alien species causing environmental impacts. 2. The document is relevant to the work of the Convention on Biological Diversity, in particular with regard to addressing (a) tools for conducting analysis for the management of invasive alien species, (b) the risks associated with trade in live alien organisms, and (c) achieving Aichi Biodiversity Target 9. 3. The document is being circulated in the form and language in which it was received by the Secretariat. * CBD/COP/14/1. The application of classical biological control for the management of established invasive alien species causing environmental impacts Summary for Policy Makers Prepared by: International Union for Conservation of Nature (IUCN), Species Survival Commission Invasive Species Specialist Group (ISSG) *Note that the full report follows on from this Summary for Policy Makers document. Summary for policy makers Convention on Biological Diversity (CBD) COP13 Decision XIII on Invasive Alien Species (IAS) recognized ‘that classical biological control can be an effective measure to manage already established invasive alien species’, and encouraged ‘Parties, other Governments and relevant organizations, when using classical biological control to manage already established invasive alien species, … [to take] into account the summary of technical considerations1’ that was annexed to the decision. -

Thistle Identification
Oklahoma Cooperative Extension Service PSS-2776 Thistle Identification January 2021 Laura Goodman Extension Rangeland Ecology Specialist Oklahoma Cooperative Extension Fact Sheets are also available on our website at: Tom Royer extension.okstate.edu Extension Entomologist Alex Rocateli can often develop. The current Thistle Law includes three of Forage Systems Extension Specialist the five species. However, all introduced thistles should be considered invasive. Oklahoma’s Noxious Weed Law, first enacted in 1994 in four counties in northeastern Oklahoma (Code 35:30-36-13) Thistles Listed in the Noxious Weed Law was amended in 1995, 1998 and 1999. The current law de- Canada thistle (Cirsium arvense) is an introduced peren- clares musk, scotch and Canada thistles to be noxious weeds nial thistle widely distributed in Nebraska and other northern and public nuisances in all counties of the state. states. At present, it does not appear to be a major threat in There are about a dozen purple-flowered spiny thistle Oklahoma. Several plants were collected in the panhandle species that occur in Oklahoma. Oklahoma’s Noxious Weed counties in the 1950s and several more in Bryan County in Law can raise concern among landowners if they do not the 1970s, but currently, no infestations are known to exist in know which thistles on their land they are required to control. the state. In a 1998 survey of noxious weeds in Meade County The purpose of this publication is to describe the introduced Kansas, north of Beaver County, Oklahoma, reported a small thistles, selected common native thistles and provide infor- infestation of Canada thistle. -

Spermatheca Structure of Cassida Atrata Fabricius, 1787 (Coleoptera: Chrysomelidae: Cassidinae) in Scanning Electron Microscope (SEM)
KSÜ Tarım ve Doğa Derg 23 (2): 396-401, 2020 KSU J. AgricNat 23 (2): 396-401, 2020 DOI:10.18016/ksutarimdoga.vi.630773 Spermatheca Structure of Cassida atrata Fabricius, 1787 (Coleoptera: Chrysomelidae: Cassidinae) in Scanning Electron Microscope (SEM) Neslihan BAL Gazi Üniversitesi Fen Fakültesi Biyoloji Bölümü https://orcid.org/0000-0001-2345-6789 : [email protected] ABSTRACT ResearchArticle It is accepted that male genitalia are not diagnostic, spermatheca are partly diagnostic within the genus Cassida Linnaeus, 1758. However, ArticleHistory so far, it appears that genital studies are based solely on stereo Received : 08.10.2019 microscopy. Ultrastructures of genitalia have been not studied except Accepted : 19.12.2019 for a few studies. In this study, female genital structure belonging to three Cassida atrata Fabricius, 1787 specimens collected from Kayseri Keywords and Niğde provinces in 1996 and 2018 from Turkey was examined for Chrysomelidae the first time in SEM in order to determine whether ultrastructural Cassida studies are useful from taxonomic point of view. Thus, new diagnostic SEM characters were obtained and it revealed that it was diagnostic for Spermatheca species in other subgenus. Photos of spermatheca taken by both SEM Turkey and stereo microscope are also given. Taramalı Elektron Mikroskobunda (SEM) Cassida atrata Fabricius, 1787’ nın SpermathecaYapısı (Coleoptera: Chrysomelidae: Cassidinae) ÖZET Araştırma Makalesi Cassida Linnaeus, 1758 cinsi içerisinde genel olarak erkek genitalinin ayırt edici olmadığı, spermateka’nın ise kısmen ayırt edici olduğu Makale Tarihçesi kabul edilmektedir. Bununla birlikte şimdiye kadar yapılan genital Geliş Tarihi : 08.10.2019 çalışmalarının sadece stereo mikroskoba dayalı olduğu Kabul Tarihi : 19.12.2019 görülmektedir. -

Integrated Noxious Weed Management Plan: US Air Force Academy and Farish Recreation Area, El Paso County, CO
Integrated Noxious Weed Management Plan US Air Force Academy and Farish Recreation Area August 2015 CNHP’s mission is to preserve the natural diversity of life by contributing the essential scientific foundation that leads to lasting conservation of Colorado's biological wealth. Colorado Natural Heritage Program Warner College of Natural Resources Colorado State University 1475 Campus Delivery Fort Collins, CO 80523 (970) 491-7331 Report Prepared for: United States Air Force Academy Department of Natural Resources Recommended Citation: Smith, P., S. S. Panjabi, and J. Handwerk. 2015. Integrated Noxious Weed Management Plan: US Air Force Academy and Farish Recreation Area, El Paso County, CO. Colorado Natural Heritage Program, Colorado State University, Fort Collins, Colorado. Front Cover: Documenting weeds at the US Air Force Academy. Photos courtesy of the Colorado Natural Heritage Program © Integrated Noxious Weed Management Plan US Air Force Academy and Farish Recreation Area El Paso County, CO Pam Smith, Susan Spackman Panjabi, and Jill Handwerk Colorado Natural Heritage Program Warner College of Natural Resources Colorado State University Fort Collins, Colorado 80523 August 2015 EXECUTIVE SUMMARY Various federal, state, and local laws, ordinances, orders, and policies require land managers to control noxious weeds. The purpose of this plan is to provide a guide to manage, in the most efficient and effective manner, the noxious weeds on the US Air Force Academy (Academy) and Farish Recreation Area (Farish) over the next 10 years (through 2025), in accordance with their respective integrated natural resources management plans. This plan pertains to the “natural” portions of the Academy and excludes highly developed areas, such as around buildings, recreation fields, and lawns.