This Article Appeared in a Journal Published by Elsevier. the Attached
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coastal Wetlunds of the Noytherrn Gua of Califurnia
AQUATIC CONSERVATION:MARINE AND FRESHWATERECOSYSTEMS Aquatic Conseru:Mar. Freshv. Ecosyst. l6: 5 28 (2006) Publishedonline in Wiley InterScience (www.interscience.wiley.com).DOI: 10.1002/aqc.68l Coastal wetlundsof the noytherrnGuA of Califurnia: inventory flnd conservutionstatus EDWARD P. GLENNO'*, PAMELA L. NAGLERU, RICHARD C. BRUSCAb and OSVEL HINOJOSA-HUERTA' " EnvironntentalResearch Laborator!-,2601 East Airport Drive, Tucson,AZ 85706, USA bAritora Sonora Desert Museum,2021 North Kinney RoacJ,Tucson, AZ 85743,USA 'Pronatura lVoroeste,Ave. Jalisco 903, Colonia Sonora, San Luis Rio Colorado, Sonora 83440. Meric'o ABSTRACT 1. Above 28"N, the coastlineof the northern Gulf of California is indented at frequent intervals by negative or inverseestuaries that are saltier at their backs than at their mouths due to the lack of freshwater inflow. These 'esteros'total over l32,ogo ha in area and encompassmangrove marshes below 29"N and saltgrass(Drsrichlis palmeri) marshes north of 29"N. An additional 6000 ha of freshwaterand brackish wetlandsare found in the Colorado River delta where fresh water entersthe intertidal zone. 2. The mangrove marshesin the Gulf of California have been afforded some degreeof protected statusin Mexico, but the northern saltgrassesteros do not have priority conservationstatus and are increasinglybecoming developmenttargets for resorts,vacation homes and aquaculture sites. 3. We conducted an inventory of the marshesusing aerial photography and satelliteimages, and evaluatedthe extent and type of developmenton eachmarsh. We reviewedthe availableliterature on the marshesto document their vegetationtypes and ecologicalfunctions in the adjacentmarine and terrestrial ecosystems. 4. Over 95"h of the mangrove marshes have been developed for shrimp farming. However, the larms are built adjacent to, rather than in, the marshes, and the mangrove stands are still mostly intact. -

Indicators for Assessing Infant and Young Child Feeding Practices Part 1 Definitions
Indicators for assessing infant and young child feeding practices PART 1 DEFINITIONS Indicators for assessing infant and young child feeding practices PART 1 DEFINITIONS Conclusions of a consensus meeting held 6–8 November 2007 in Washington, DC, USA WHO Library Cataloguing-in-Publication Data Indicators for assessing infant and young child feeding practices : conclusions of a consensus meeting held 6–8 November 2007 in Washington D.C., USA. 1.Infant nutrition. 2.Breast feeding. 3.Bottle feeding. 4.Feeding behavior. 5.Indicators. I.World Health Organization. Dept. of Child and Adolescent Health and Development. ISBN 978 92 4 159666 4 (NLM classification: WS 120) © World Health Organization 2008 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps rep- resent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or rec- ommended by the World Health Organization in preference to others of a similar nature that are not mentioned. -

Agronomy of Halophytes As Constructive Use of Saline Systems
Agronomy of Halophytes as Constructive Use of Saline Systems Item Type text; Electronic Dissertation Authors Bresdin, Cylphine Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 07/10/2021 03:23:03 Link to Item http://hdl.handle.net/10150/577318 AGRONOMY OF HALOPHYTES AS CONSTRUCTIVE USE OF SALINE SYSTEMS by Cylphine Bresdin A Dissertation Submitted to the Faculty of the department of SOIL, WATER AND ENVIRONMENTAL ScIENCES In Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY WITH A MAJOR IN ENVIRONMENTAL SCIENCE In the Graduate College ThE UNIVERSITY OF ARIZONA 2015 1 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Cylphine Bresdin, titled Agronomy of Halophytes as Constructive Use of Saline Systems and recommend that it be accepted as fulfilling the dissertation requirement for the Degree of Doctor of Philosophy. _____________________________________________________ Date: 7/29/2015 Edward Glenn _____________________________________________________ Date: 7/29/2015 Janick Artiola _____________________________________________________ Date: 7/29/2015 Kevin Fitzsimmons _____________________________________________________ Date: 7/29/2015 Margaret Livingston Final approval and acceptance of this dissertation is contingent upon the candidate’s submission of the final copies of the dissertation to the Graduate College. I hereby certify that I have read this dissertation prepared under my direction and recommend that it be accepted as fulfilling the dissertation requirement. -

Minimum Dietary Diversity for Women
MINIMUM DIETARY DIVERSITY FOR WOMEN An updated guide for measurement: from collection to action MINIMUM DIETARY DIVERSITY FOR WOMEN An updated guide for measurement: from collection to action Food and Agriculture Organization of the United Nations Rome, 2021 Required citation: FAO. 2021. Minimum dietary diversity for women. Rome. https://doi.org/10.4060/cb3434en The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. ISBN 978-92-5-133993-0 © FAO, 2021 Some rights reserved. This work is made available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo/legalcode). Under the terms of this licence, this work may be copied, redistributed and adapted for non-commercial purposes, provided that the work is appropriately cited. In any use of this work, there should be no suggestion that FAO endorses any specific organization, products or services. The use of the FAO logo is not permitted. If the work is adapted, then it must be licensed under the same or equivalent Creative Commons licence. -

Indicators for Assessing Infant and Young Child Feeding Practices Definitions and Measurement Methods
Indicators for assessing infant and young child feeding practices Definitions and measurement methods Indicators for assessing infant and young child feeding practices Definitions and measurement methods Indicators for assessing infant and young child feeding practices: definitions and measurement methods ISBN (WHO) 978-92-4-001838-9 (electronic version) ISBN (WHO) 978-92-4-001839-6 (print version) © World Health Organization and the United Nations Children’s Fund (UNICEF), 2021 This joint report reflects the activities of the World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO or UNICEF endorses any specific organization, products or services. The unauthorized use of the WHO or UNICEF names or logos is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO) or the United Nations Children’s Fund (UNICEF). Neither WHO nor UNICEF are responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. -

A Molecular Phylogeny and Classification of the Cynodonteae
TAXON 65 (6) • December 2016: 1263–1287 Peterson & al. • Phylogeny and classification of the Cynodonteae A molecular phylogeny and classification of the Cynodonteae (Poaceae: Chloridoideae) with four new genera: Orthacanthus, Triplasiella, Tripogonella, and Zaqiqah; three new subtribes: Dactylocteniinae, Orininae, and Zaqiqahinae; and a subgeneric classification of Distichlis Paul M. Peterson,1 Konstantin Romaschenko,1,2 & Yolanda Herrera Arrieta3 1 Smithsonian Institution, Department of Botany, National Museum of Natural History, Washington, D.C. 20013-7012, U.S.A. 2 M.G. Kholodny Institute of Botany, National Academy of Sciences, Kiev 01601, Ukraine 3 Instituto Politécnico Nacional, CIIDIR Unidad Durango-COFAA, Durango, C.P. 34220, Mexico Author for correspondence: Paul M. Peterson, [email protected] ORCID PMP, http://orcid.org/0000-0001-9405-5528; KR, http://orcid.org/0000-0002-7248-4193 DOI https://doi.org/10.12705/656.4 Abstract Morphologically, the tribe Cynodonteae is a diverse group of grasses containing about 839 species in 96 genera and 18 subtribes, found primarily in Africa, Asia, Australia, and the Americas. Because the classification of these genera and spe cies has been poorly understood, we conducted a phylogenetic analysis on 213 species (389 samples) in the Cynodonteae using sequence data from seven plastid regions (rps16-trnK spacer, rps16 intron, rpoC2, rpl32-trnL spacer, ndhF, ndhA intron, ccsA) and the nuclear ribosomal internal transcribed spacer regions (ITS 1 & 2) to infer evolutionary relationships and refine the -
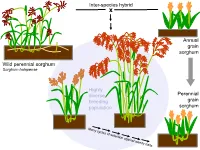
Variation in Plant Form in Recurrent Selection Populations of Kansas Rosinseed
Inter-species hybrid x Annual grain sorghum Wild perennial sorghum Sorghum halapense Highly diverse Perennial breeding grain population sorghum 160 cm BULK06-10 perennial legumes 1. The Land Institute is domesticating Illinois bundleflower (IBF) – Adapted to Great Plains conditions – Nitrogen fixation and seed protein content similar to soybean Dehiscent Indehiscent Pod dropping score (0=marcescent, 2=highly deciduous) 2008 families: 0 0.5 1 2 Indehiscent families 11 13 36 48 Dehiscent families 18 2 2 1 Pod dropping score (0=marcescent, 2=highly deciduous) 2008 families: 0 0.5 1 2 Indehiscent families 11 13 36 48 Dehiscent families 18 2 2 1 2. Existing forage legumes as nitrogen source for perennial cereals Mowed legume intercrop Multi-row mower tractor attachment Late spring: Mow between each row of cereal Early summer: Mowing triggers legume to drop fine roots; nitrogen from decomposing roots and leaves is taken up by the cereal crop 3. Existing perennial grain legumes – but usually grown as annuals • pigeonpea • lab-lab • runner bean • Lima bean Pigeon Pea •Deeply-rooted, perennial •4.92 million hectares worldwide (3.58 million in India alone) •Average yield 898 kg/ha ―The pigeonpea plants, especially of the perennial varieties, have a strong root system, which helps hold the soil on sloping hillsides. ― ―’Pigeonpea has been found to be very successful in covering the soil and reducing soil erosion," says Dr Zong Xuxiao, from the Chinese Academy of Agricultural Sciences at Beijing.’‖ http://www.cgiar.org/newsroom/releases/news.asp?idnews=536 -

Flora of Southwestern Arizona
Felger, R.S., S. Rutman, and J. Malusa. 2014. Ajo Peak to Tinajas Altas: A flora of southwestern Arizona. Part 6. Poaceae – grass family. Phytoneuron 2014-35: 1–139. Published 17 March 2014. ISSN 2153 733X AJO PEAK TO TINAJAS ALTAS: A FLORA OF SOUTHWESTERN ARIZONA Part 6. POACEAE – GRASS FAMILY RICHARD STEPHEN FELGER Herbarium, University of Arizona Tucson, Arizona 85721 & Sky Island Alliance P.O. Box 41165, Tucson, Arizona 85717 *Author for correspondence: [email protected] SUSAN RUTMAN 90 West 10th Street Ajo, Arizona 85321 JIM MALUSA School of Natural Resources and the Environment University of Arizona Tucson, Arizona 85721 [email protected] ABSTRACT A floristic account is provided for the grass family as part of the vascular plant flora of the contiguous protected areas of Organ Pipe Cactus National Monument, Cabeza Prieta National Wildlife Refuge, and the Tinajas Altas Region in southwestern Arizona. This is the second largest family in the flora area after Asteraceae. A total of 97 taxa in 46 genera of grasses are included in this publication, which includes ones established and reproducing in the modern flora (86 taxa in 43 genera), some occurring at the margins of the flora area or no long known from the area, and ice age fossils. At least 28 taxa are known by fossils recovered from packrat middens, five of which have not been found in the modern flora: little barley ( Hordeum pusillum ), cliff muhly ( Muhlenbergia polycaulis ), Paspalum sp., mutton bluegrass ( Poa fendleriana ), and bulb panic grass ( Zuloagaea bulbosa ). Non-native grasses are represented by 27 species, or 28% of the modern grass flora. -

Coastal Wetlands of the Northern Gulf of California: Inventory and Conservation Status
AQUATIC CONSERVATION: MARINE AND FRESHWATER ECOSYSTEMS Aquatic Conserv: Mar. Freshw. Ecosyst. 16: 5–28 (2006) Published online in Wiley InterScience (www.interscience.wiley.com). DOI: 10.1002/aqc.681 Coastal wetlands of the northern Gulf of California: inventory and conservation status EDWARD P. GLENNa,*, PAMELA L. NAGLERa, RICHARD C. BRUSCAb and OSVEL HINOJOSA-HUERTAc a Environmental Research Laboratory, 2601 East Airport Drive, Tucson, AZ 85706, USA b Arizona–Sonora Desert Museum, 2021 North Kinney Road, Tucson, AZ 85743, USA c Pronatura Noroeste, Ave. Jalisco 903, Colonia Sonora, San Luis R!ııo Colorado, Sonora 83440, Mexico ABSTRACT 1. Above 288N, the coastline of the northern Gulf of California is indented at frequent intervals by negative or inverse estuaries that are saltier at their backs than at their mouths due to the lack of freshwater inflow. These ‘esteros’ total over 215 000 ha in area and encompass mangrove marshes below 298N and saltgrass (Distichlis palmeri) marshes north of 298N. An additional 6000 ha of freshwater and brackish wetlands are found in the Colorado River delta where fresh water enters the intertidal zone. 2. The mangrove marshes in the Gulf of California have been afforded some degree of protected status in Mexico, but the northern saltgrass esteros do not have priority conservation status and are increasingly becoming development targets for resorts, vacation homes and aquaculture sites. 3. We conducted an inventory of the marshes using aerial photography and satellite images, and evaluated the extent and type of development on each marsh. We reviewed the available literature on the marshes to document their vegetation types and ecological functions in the adjacent marine and terrestrial ecosystems. -

FEIS Citation Retrieval System Keywords
FEIS Citation Retrieval System Keywords 29,958 entries as KEYWORD (PARENT) Descriptive phrase AB (CANADA) Alberta ABEESC (PLANTS) Abelmoschus esculentus, okra ABEGRA (PLANTS) Abelia × grandiflora [chinensis × uniflora], glossy abelia ABERT'S SQUIRREL (MAMMALS) Sciurus alberti ABERT'S TOWHEE (BIRDS) Pipilo aberti ABIABI (BRYOPHYTES) Abietinella abietina, abietinella moss ABIALB (PLANTS) Abies alba, European silver fir ABIAMA (PLANTS) Abies amabilis, Pacific silver fir ABIBAL (PLANTS) Abies balsamea, balsam fir ABIBIF (PLANTS) Abies bifolia, subalpine fir ABIBRA (PLANTS) Abies bracteata, bristlecone fir ABICON (PLANTS) Abies concolor, white fir ABICONC (ABICON) Abies concolor var. concolor, white fir ABICONL (ABICON) Abies concolor var. lowiana, Rocky Mountain white fir ABIDUR (PLANTS) Abies durangensis, Coahuila fir ABIES SPP. (PLANTS) firs ABIETINELLA SPP. (BRYOPHYTES) Abietinella spp., mosses ABIFIR (PLANTS) Abies firma, Japanese fir ABIFRA (PLANTS) Abies fraseri, Fraser fir ABIGRA (PLANTS) Abies grandis, grand fir ABIHOL (PLANTS) Abies holophylla, Manchurian fir ABIHOM (PLANTS) Abies homolepis, Nikko fir ABILAS (PLANTS) Abies lasiocarpa, subalpine fir ABILASA (ABILAS) Abies lasiocarpa var. arizonica, corkbark fir ABILASB (ABILAS) Abies lasiocarpa var. bifolia, subalpine fir ABILASL (ABILAS) Abies lasiocarpa var. lasiocarpa, subalpine fir ABILOW (PLANTS) Abies lowiana, Rocky Mountain white fir ABIMAG (PLANTS) Abies magnifica, California red fir ABIMAGM (ABIMAG) Abies magnifica var. magnifica, California red fir ABIMAGS (ABIMAG) Abies -

Drought-Tolerant Options for Southwest Agriculture: Grasses, Grains, and Legumes
May 2021 Applied Economics/2021-04pr Drought-Tolerant Options for Southwest Agriculture: Grasses, Grains, and Legumes Emily Rice, Extension Intern, Department of Applied Economics Kynda Curtis, Professor and Extension Specialist, Department of Applied Economics Introduction The arid Southwest has been heavily impacted by drought and climate change. Temperatures in the region rose 2 degrees Fahrenheit in the last century and are expected to rise 3 to 9 degrees by the end of the century (Climate Impacts in the Southwest, 2016). The Southwest states are some of the driest in the nation. From 2000 to 2020, 65% of the state of Utah experienced abnormally dry conditions, 78% of Arizona, 69% of New Mexico, and 71% of Nevada. Water shortages are also common, especially in the late summer when water restrictions are often imposed. Drought levels of this magnitude damage pastures and crops, leading to economic losses in agriculture. 1 United Nations Environment Report in 2006 stated that the grass was “a strong candidate for a major global food crop and could become this desert’s greatest gift to the world” (Distichlis palmeri, n.d.). Its flour tastes sweet and is a gluten-free alternative. Saltgrass 2 and helps minimize water loss, thus giving it better drought tolerance (Carter et al., 1989). Pearl Millet (Pennisetum glaucum) According to the United States Department of Agriculture’s (USDA’s) Plant Guide, pearl millet is best grown in zone 5 (USDA, 2016) and is the highest-yielding type of millet. It’s also a suitable feed for livestock as it does not produce prussic acid (Pearl Millet, 2021). -
UNIVERSIDAD NACIONAL DEL LITORAL Facultad De Bioquímica Y Ciencias Biológicas
UNIVERSIDAD NACIONAL DEL LITORAL Facultad de Bioquímica y Ciencias Biológicas Tesis para la obtención del Grado Académico de Doctor en Ciencias Biológicas DIVERSIDAD Y EVOLUCIÓN DE LAS INFLORESCENCIAS EN LAS SUBTRIBUS MÁS DERIVADAS DE CYNODONTTEAE (CHLORIDOIDEAE-POACEAE) Lic. Vanesa Pilatti Director de Tesis: Dr. Abelardo Vegetti Co-directora de Tesis: Dra. Renata Reinheimer Morfología Vegetal - Facultad de Ciencias Agrarias Universidad Nacional del Litoral -2016- A Gonzalo… a mi querida familia… y a mis abuelos… AGRADEZCO… …a la Facultad de Bioquímica y Ciencias Biológicas (FBCB-UNL) por permitirme realizar el “Doctorado en Ciencias Biológicas”; y especialmente a todo el grupo de personas, que siempre de manera amable y eficaz pudieron resolver todas mis inquietudes. …a la Facultad de Ciencias Agrarias (FCA-UNL), por haberme abierto sus puertas, y facilitado herramientas e infraestructura para la realización de esta tesis. …al Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), por haberme otorgado las becas de posgrado (“Tipo I” y “Tipo II”) que me permitieron dedicarme exclusivamente a la tesis. … a Abelardo Vegetti, por haberme dado la posibilidad de integrar su grupo de trabajo y poder trabajar con las plantas, por transmitirme todos su conocimiento sobre el mundo de las inflorescencias y la morfología vegetal, por confiar siempre en mí, por su buena predisposición siempre, por su apoyo y cariño. … a Renata Reinheimer, por haber aceptado ser mi co-directora, por su actitud reflexiva y crítica, por todo el esfuerzo y dedicación que me brindo principalmente en este último tiempo, por sus consejos valiosos a lo largo del doctorado y de la escritura de la tesis.