Perennial Wheat Breeding: Current Germplasm and a Way Forward for Breeding and Global Cooperation
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

'Is the Future of Agriculture Perennial?'
‘Is the Future of Agriculture Perennial?’ LUND, 6-10TH OF MAY 2019 2 ABSTRACTS FOR ‘IS THE FUTURE OF AGRICULTURE PERENNIAL?’, LUND, 6-10TH OF MAY 2019 Abstracts Keynote talks Tuesday 7/5 Wes Jackson, The Land Institute Nature Systems Agriculture and the Need for a Creaturely World View Natural Systems Agriculture began less than a year after our 1976 beginning when my students and I took a field trip to a native Kansas Prairie. Noting the contrast between nature’s prairie and annual grain monocultures the reality of nature’s wisdom and the failure of human cleverness was clear. Perennial grain polycultures research began, but with full awareness that science is embedded in dominating social organizations which must be well understood if we are to address the countless problems in our climate changing ecosphere. To that end I will give a brief history of our origins and argue for a information rich creaturely world view to replace the industrial mind. John Head, Kansas University Is the Future of Agroecological Governance also Perennial? In addition to the scientific innovations that will allow us to give an affirmative answer to the central question of this conference – “Is the Future of Agriculture Perennial?” – we must also consider and design legal and institutional innovations, especially at the global level, that can facilitate an effective transformation from modern extractive agriculture to agroecological husbandry. These reforms in agroecological governance should give special emphasis to (i) reconceptualizing state sovereignty to reflect 21st- century realities and (ii) introducing new legal entities (“eco-states”) with authority to manage agroecological matters in ways that will address the soil and climate crises. -

Perennial Grain Crop Roots and Nitrogen Management Shape Soil Food Webs T and Soil Carbon Dynamics ∗ Christine D
Soil Biology and Biochemistry 137 (2019) 107573 Contents lists available at ScienceDirect Soil Biology and Biochemistry journal homepage: www.elsevier.com/locate/soilbio Perennial grain crop roots and nitrogen management shape soil food webs T and soil carbon dynamics ∗ Christine D. Sprungera, , Steven W. Culmana, Ariane L. Peraltab, S. Tianna DuPontc, Jay T. Lennond, Sieglinde S. Snappe a School of Environment and Natural Resources, The Ohio State University, Wooster, OH, 44691, USA b Department of Biology, East Carolina University, Greenville, NC, 27858, USA c Tree Fruit Research and Extension, Washington State University, Wenatchee, WA, 98801, USA d Department of Biology, Indiana University, Bloomington, IN, 47405, USA e Department of Plant, Soil, and Microbial Sciences, Michigan State University, East Lansing, MI, 48824, USA ARTICLE INFO ABSTRACT Keywords: Perennial grain crops may confer greater ecosystem services relative to annual row crop systems due to their Organic matter extensive roots systems and year-round ground cover. However, less is known about the extent to which per- Root biomass ennial grain crops affect food web dynamics and soil carbon (C) cycling over time. Furthermore, manyme- Nematodes chanistic questions remain regarding the influence of root quantity and quality on soil biological communities Bacteria and C cycling function. In this study, we quantified root biomass and quality, bacterial and nematode community Soil food webs structure, and labile soil C pools of perennial intermediate wheatgrass [Thinopyrum intermedium (Host) Buckworth and Dewey] and annual winter wheat (Triticum aes L.) across three nitrogen (N) management systems (Organic, Low inorganic N, High inorganic N). After 4 years, the perennial grain crop had significantly greater root quantity and permanganate oxidizable carbon (POXC) relative to annual wheat. -

Annual Report 2019 About the Land Institute
Annual Report 2019 About The Land Institute Since 1976, The Land Institute has worked to transform grain agriculture globally from a system of extractive domination to one of generative care. Nature is our measure and from nature we are given two values that are key to a truly regenerative agriculture and to a healthy, just society in equilibrium with the earth of which it is a part: perenniality and diversity. Our team of plant breeders and ecologists, together with collaborators on six continents, are working to develop perennial grains, pulses, and oilseed bearing plants to be grown in ecologically intensified, diverse crop mixtures. When realized, this new agricultural system promises to hold and restore soil, produce ample food, require less fossil fuels, conserve water, and mitigate and endure the impacts of climate change. In tandem, we are working to extend the insights of this new agricultural system to aid human communities to live in just relationships within an ecospheric standard. The Land Institute is a nonprofit 501(c)(3) research and education organization funded by charitable contributions from individuals, organizations, and private foundations. Cover: Megan Gladbach, a perennial sorghum technician at The Land Institute, battles weeds with a hoe in a field of sorghum. The Institute is breeding sorghum to be a perennial food crop. Above: Crystal Ma, left, and Sienna Polk (interns) carry bags of harvested perennial wheat to the edge of a breed- ing plot for pickup. The wheat will later be threshed and analyzed in the lab. Looking on is Piyush Labhsetwar, a perennial wheat research technician. -

Coastal Wetlunds of the Noytherrn Gua of Califurnia
AQUATIC CONSERVATION:MARINE AND FRESHWATERECOSYSTEMS Aquatic Conseru:Mar. Freshv. Ecosyst. l6: 5 28 (2006) Publishedonline in Wiley InterScience (www.interscience.wiley.com).DOI: 10.1002/aqc.68l Coastal wetlundsof the noytherrnGuA of Califurnia: inventory flnd conservutionstatus EDWARD P. GLENNO'*, PAMELA L. NAGLERU, RICHARD C. BRUSCAb and OSVEL HINOJOSA-HUERTA' " EnvironntentalResearch Laborator!-,2601 East Airport Drive, Tucson,AZ 85706, USA bAritora Sonora Desert Museum,2021 North Kinney RoacJ,Tucson, AZ 85743,USA 'Pronatura lVoroeste,Ave. Jalisco 903, Colonia Sonora, San Luis Rio Colorado, Sonora 83440. Meric'o ABSTRACT 1. Above 28"N, the coastlineof the northern Gulf of California is indented at frequent intervals by negative or inverseestuaries that are saltier at their backs than at their mouths due to the lack of freshwater inflow. These 'esteros'total over l32,ogo ha in area and encompassmangrove marshes below 29"N and saltgrass(Drsrichlis palmeri) marshes north of 29"N. An additional 6000 ha of freshwaterand brackish wetlandsare found in the Colorado River delta where fresh water entersthe intertidal zone. 2. The mangrove marshesin the Gulf of California have been afforded some degreeof protected statusin Mexico, but the northern saltgrassesteros do not have priority conservationstatus and are increasinglybecoming developmenttargets for resorts,vacation homes and aquaculture sites. 3. We conducted an inventory of the marshesusing aerial photography and satelliteimages, and evaluatedthe extent and type of developmenton eachmarsh. We reviewedthe availableliterature on the marshesto document their vegetationtypes and ecologicalfunctions in the adjacentmarine and terrestrial ecosystems. 4. Over 95"h of the mangrove marshes have been developed for shrimp farming. However, the larms are built adjacent to, rather than in, the marshes, and the mangrove stands are still mostly intact. -

Indicators for Assessing Infant and Young Child Feeding Practices Part 1 Definitions
Indicators for assessing infant and young child feeding practices PART 1 DEFINITIONS Indicators for assessing infant and young child feeding practices PART 1 DEFINITIONS Conclusions of a consensus meeting held 6–8 November 2007 in Washington, DC, USA WHO Library Cataloguing-in-Publication Data Indicators for assessing infant and young child feeding practices : conclusions of a consensus meeting held 6–8 November 2007 in Washington D.C., USA. 1.Infant nutrition. 2.Breast feeding. 3.Bottle feeding. 4.Feeding behavior. 5.Indicators. I.World Health Organization. Dept. of Child and Adolescent Health and Development. ISBN 978 92 4 159666 4 (NLM classification: WS 120) © World Health Organization 2008 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps rep- resent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or rec- ommended by the World Health Organization in preference to others of a similar nature that are not mentioned. -

Agronomy of Halophytes As Constructive Use of Saline Systems
Agronomy of Halophytes as Constructive Use of Saline Systems Item Type text; Electronic Dissertation Authors Bresdin, Cylphine Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 07/10/2021 03:23:03 Link to Item http://hdl.handle.net/10150/577318 AGRONOMY OF HALOPHYTES AS CONSTRUCTIVE USE OF SALINE SYSTEMS by Cylphine Bresdin A Dissertation Submitted to the Faculty of the department of SOIL, WATER AND ENVIRONMENTAL ScIENCES In Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY WITH A MAJOR IN ENVIRONMENTAL SCIENCE In the Graduate College ThE UNIVERSITY OF ARIZONA 2015 1 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Cylphine Bresdin, titled Agronomy of Halophytes as Constructive Use of Saline Systems and recommend that it be accepted as fulfilling the dissertation requirement for the Degree of Doctor of Philosophy. _____________________________________________________ Date: 7/29/2015 Edward Glenn _____________________________________________________ Date: 7/29/2015 Janick Artiola _____________________________________________________ Date: 7/29/2015 Kevin Fitzsimmons _____________________________________________________ Date: 7/29/2015 Margaret Livingston Final approval and acceptance of this dissertation is contingent upon the candidate’s submission of the final copies of the dissertation to the Graduate College. I hereby certify that I have read this dissertation prepared under my direction and recommend that it be accepted as fulfilling the dissertation requirement. -

Minimum Dietary Diversity for Women
MINIMUM DIETARY DIVERSITY FOR WOMEN An updated guide for measurement: from collection to action MINIMUM DIETARY DIVERSITY FOR WOMEN An updated guide for measurement: from collection to action Food and Agriculture Organization of the United Nations Rome, 2021 Required citation: FAO. 2021. Minimum dietary diversity for women. Rome. https://doi.org/10.4060/cb3434en The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. ISBN 978-92-5-133993-0 © FAO, 2021 Some rights reserved. This work is made available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo/legalcode). Under the terms of this licence, this work may be copied, redistributed and adapted for non-commercial purposes, provided that the work is appropriately cited. In any use of this work, there should be no suggestion that FAO endorses any specific organization, products or services. The use of the FAO logo is not permitted. If the work is adapted, then it must be licensed under the same or equivalent Creative Commons licence. -

Rye, Triticale, and Intermediate Wheatgrass: Recent Updates in Research, Plant Breeding, and Their Common Uses
Iowa State University Capstones, Theses and Creative Components Dissertations Summer 2020 Rye, triticale, and intermediate wheatgrass: Recent updates in research, plant breeding, and their common uses Jeremiah Menefee Follow this and additional works at: https://lib.dr.iastate.edu/creativecomponents Part of the Agriculture Commons Recommended Citation Menefee, Jeremiah, "Rye, triticale, and intermediate wheatgrass: Recent updates in research, plant breeding, and their common uses" (2020). Creative Components. 601. https://lib.dr.iastate.edu/creativecomponents/601 This Creative Component is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Creative Components by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Rye, triticale, and intermediate wheatgrass: Recent updates in research, plant breeding, and their common uses by Jeremiah Menefee A creative component submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Agronomy Program of Study Committee: Arti Singh, Major Professor Thomas Lubberstedt Iowa State University Ames, Iowa June, 2020 Copyright © Jeremiah Menefee, 2020. All rights reserved. ii DEDICATION This creative component is dedicated to my wife, Danielle Menefee. Thank you for putting up with me! iii TABLE OF CONTENTS Page ACKNOWLEDGMENTS ........................................................................................ -

Indicators for Assessing Infant and Young Child Feeding Practices Definitions and Measurement Methods
Indicators for assessing infant and young child feeding practices Definitions and measurement methods Indicators for assessing infant and young child feeding practices Definitions and measurement methods Indicators for assessing infant and young child feeding practices: definitions and measurement methods ISBN (WHO) 978-92-4-001838-9 (electronic version) ISBN (WHO) 978-92-4-001839-6 (print version) © World Health Organization and the United Nations Children’s Fund (UNICEF), 2021 This joint report reflects the activities of the World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO or UNICEF endorses any specific organization, products or services. The unauthorized use of the WHO or UNICEF names or logos is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO) or the United Nations Children’s Fund (UNICEF). Neither WHO nor UNICEF are responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. -

Approaches to Managing Intermediate Wheatgrass for Dual-Use Forage and Kernza® Perennial Grain Production
Approaches to Managing Intermediate Wheatgrass for Dual-Use Forage and Kernza® Perennial Grain Production Last Updated: March, 2019 This is an evolving document and will be updated as research and on-farm experience continue. Future versions will be made available on kernza.com Images: Jérémie Favre (L), Mette Nielsen (R) A note on Kernza® intermediate wheatgrass development: This document includes suggestions for how to grow Kernza® intermediate wheatgrass for dual-use forage and grain production* in the U.S. Upper Midwest. Intermediate wheatgrass (scientific name Thinopyrum intermedium) is a perennial grass species related to wheat. While it is commonly planted in the U.S. as a forage grass, breeding programs at The Land Institute and the University of Minnesota have used the species to develop a promising new perennial grain crop. Selection for grain size, disease resistance, and other traits have advanced to the point that early-adopter farmers are now beginning to experiment with planting the crop. This document is meant to be a resource created for, and in partnership with, those farmer innovators. Kernza® is the trademarked name of the edible perennial grain harvested from the intermediate wheatgrass plant. The trademark is held by The Land Institute, a research-based non-profit organization in Salina, Kansas. Additional history can be found at kernza.org and forevergreen.umn.edu. Throughout this document the plant/crop is referred to as “Kernza intermediate wheatgrass”. Kernza intermediate wheatgrass is actively being researched and developed at research institutions and on farms. This is a new and experimental grain crop, and markets are in the early stages of development. -

A Molecular Phylogeny and Classification of the Cynodonteae
TAXON 65 (6) • December 2016: 1263–1287 Peterson & al. • Phylogeny and classification of the Cynodonteae A molecular phylogeny and classification of the Cynodonteae (Poaceae: Chloridoideae) with four new genera: Orthacanthus, Triplasiella, Tripogonella, and Zaqiqah; three new subtribes: Dactylocteniinae, Orininae, and Zaqiqahinae; and a subgeneric classification of Distichlis Paul M. Peterson,1 Konstantin Romaschenko,1,2 & Yolanda Herrera Arrieta3 1 Smithsonian Institution, Department of Botany, National Museum of Natural History, Washington, D.C. 20013-7012, U.S.A. 2 M.G. Kholodny Institute of Botany, National Academy of Sciences, Kiev 01601, Ukraine 3 Instituto Politécnico Nacional, CIIDIR Unidad Durango-COFAA, Durango, C.P. 34220, Mexico Author for correspondence: Paul M. Peterson, [email protected] ORCID PMP, http://orcid.org/0000-0001-9405-5528; KR, http://orcid.org/0000-0002-7248-4193 DOI https://doi.org/10.12705/656.4 Abstract Morphologically, the tribe Cynodonteae is a diverse group of grasses containing about 839 species in 96 genera and 18 subtribes, found primarily in Africa, Asia, Australia, and the Americas. Because the classification of these genera and spe cies has been poorly understood, we conducted a phylogenetic analysis on 213 species (389 samples) in the Cynodonteae using sequence data from seven plastid regions (rps16-trnK spacer, rps16 intron, rpoC2, rpl32-trnL spacer, ndhF, ndhA intron, ccsA) and the nuclear ribosomal internal transcribed spacer regions (ITS 1 & 2) to infer evolutionary relationships and refine the -
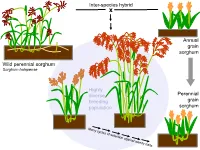
Variation in Plant Form in Recurrent Selection Populations of Kansas Rosinseed
Inter-species hybrid x Annual grain sorghum Wild perennial sorghum Sorghum halapense Highly diverse Perennial breeding grain population sorghum 160 cm BULK06-10 perennial legumes 1. The Land Institute is domesticating Illinois bundleflower (IBF) – Adapted to Great Plains conditions – Nitrogen fixation and seed protein content similar to soybean Dehiscent Indehiscent Pod dropping score (0=marcescent, 2=highly deciduous) 2008 families: 0 0.5 1 2 Indehiscent families 11 13 36 48 Dehiscent families 18 2 2 1 Pod dropping score (0=marcescent, 2=highly deciduous) 2008 families: 0 0.5 1 2 Indehiscent families 11 13 36 48 Dehiscent families 18 2 2 1 2. Existing forage legumes as nitrogen source for perennial cereals Mowed legume intercrop Multi-row mower tractor attachment Late spring: Mow between each row of cereal Early summer: Mowing triggers legume to drop fine roots; nitrogen from decomposing roots and leaves is taken up by the cereal crop 3. Existing perennial grain legumes – but usually grown as annuals • pigeonpea • lab-lab • runner bean • Lima bean Pigeon Pea •Deeply-rooted, perennial •4.92 million hectares worldwide (3.58 million in India alone) •Average yield 898 kg/ha ―The pigeonpea plants, especially of the perennial varieties, have a strong root system, which helps hold the soil on sloping hillsides. ― ―’Pigeonpea has been found to be very successful in covering the soil and reducing soil erosion," says Dr Zong Xuxiao, from the Chinese Academy of Agricultural Sciences at Beijing.’‖ http://www.cgiar.org/newsroom/releases/news.asp?idnews=536