Entry Point Related Outcome in Antegrade Femoral Nailing Experimental and Clinical Studies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Anatomy of the Lower Extremity
Государственное бюджетное образовательное учреждение высшего профессионального образования «Иркутский государственный медицинский университет» Министерства здравоохранения Российской Федерации Department of Operative Surgery and Topographic Anatomy Clinical anatomy of the lower extremity Teaching aid Иркутск ИГМУ 2016 УДК [617.58 + 611.728](075.8) ББК 54.578.4я73. К 49 Recommended by faculty methodological council of medical department of SBEI HE ISMU The Ministry of Health of The Russian Federation as a training manual for independent work of foreign students from medical faculty, faculty of pediatrics, faculty of dentistry, protocol № 01.02.2016. Authors: G.I. Songolov - associate professor, Head of Department of Operative Surgery and Topographic Anatomy, PhD, MD SBEI HE ISMU The Ministry of Health of The Russian Federation. O. P.Galeeva - associate professor of Department of Operative Surgery and Topographic Anatomy, MD, PhD SBEI HE ISMU The Ministry of Health of The Russian Federation. A.A. Yudin - assistant of department of Operative Surgery and Topographic Anatomy SBEI HE ISMU The Ministry of Health of The Russian Federation. S. N. Redkov – assistant of department of Operative Surgery and Topographic Anatomy SBEI HE ISMU THE Ministry of Health of The Russian Federation. Reviewers: E.V. Gvildis - head of department of foreign languages with the course of the Latin and Russian as foreign languages of SBEI HE ISMU The Ministry of Health of The Russian Federation, PhD, L.V. Sorokina - associate Professor of Department of Anesthesiology and Reanimation at ISMU, PhD, MD Songolov G.I K49 Clinical anatomy of lower extremity: teaching aid / Songolov G.I, Galeeva O.P, Redkov S.N, Yudin, A.A.; State budget educational institution of higher education of the Ministry of Health and Social Development of the Russian Federation; "Irkutsk State Medical University" of the Ministry of Health and Social Development of the Russian Federation Irkutsk ISMU, 2016, 45 p. -

Thigh Muscles
Lecture 14 THIGH MUSCLES ANTERIOR and Medial COMPARTMENT BY Dr Farooq Khan Aurakzai PMC Dated: 03.08.2021 INTRODUCTION What are the muscle compartments? The limbs can be divided into segments. If these segments are cut transversely, it is apparent that they are divided into multiple sections. These are called fascial compartments, and are formed by tough connective tissue septa. Compartments are groupings of muscles, nerves, and blood vessels in your arms and legs. INTRODUCTION to the thigh Muscles The musculature of the thigh can be split into three sections by intermuscular septas in to; Anterior compartment Medial compartment and Posterior compartment. Each compartment has a distinct innervation and function. • The Anterior compartment muscle are the flexors of hip and extensors of knee. • The Medial compartment muscle are adductors of thigh. • The Posterior compartment muscle are extensor of hip and flexors of knee. Anterior Muscles of thigh The muscles in the anterior compartment of the thigh are innervated by the femoral nerve (L2-L4), and as a general rule, act to extend the leg at the knee joint. There are three major muscles in the anterior thigh –: • The pectineus, • Sartorius and • Quadriceps femoris. In addition to these, the end of the iliopsoas muscle passes into the anterior compartment. ANTERIOR COMPARTMENT MUSCLE 1. SARTORIUS Is a long strap like and the most superficial muscle of the thigh descends obliquely Is making one of the tendon of Pes anserinus . In the upper 1/3 of the thigh the med margin of it makes the lat margin of Femoral triangle. Origin: Anterior superior iliac spine. -

Hip Joint: Embryology, Anatomy and Biomechanics
ISSN: 2574-1241 Volume 5- Issue 4: 2018 DOI: 10.26717/BJSTR.2018.12.002267 Ahmed Zaghloul. Biomed J Sci & Tech Res Review Article Open Access Hip Joint: Embryology, Anatomy and Biomechanics Ahmed Zaghloul1* and Elalfy M Mohamed2 1Assistant Lecturer, Department of Orthopedic Surgery and Traumatology, Faculty of Medicine, Mansoura University, Egypt 2Domenstrator, Department of Orthopedic Surgery and Traumatology, Faculty of Medicine, Mansoura University, Egypt Received: : December 11, 2018; Published: : December 20, 2018 *Corresponding author: Ahmed Zaghloul, Assistant Lecturer, Department of Orthopedic Surgery and Traumatology, Faculty of Medicine, Mansoura University, Egypt Abstract Introduction: Hip joint is matchless developmentally, anatomically and physiologically. It avails both mobility and stability. As the structural linkage between the axial skeleton and lower limbs, it plays a pivotal role in transmitting forces from the ground up and carrying forces from the trunk, head, neck and upper limbs down. This Article reviews the embryology, anatomy and biomechanics of the hip to give a hand in diagnosis, evaluation and treatment of hip disorders. Discussion: Exact knowledge about development, anatomy and biomechanics of hip joint has been a topic of interest and debate in literature dating back to at least middle of 18th century, as Hip joint is liable for several number of pediatric and adult disorders. The proper acting of the hip counts on the normal development and congruence of the articular surfaces of the femoral head (ball) and the acetabulum (socket). It withstands enormous loads from muscular, gravitational and joint reaction forces inherent in weight bearing. Conclusion: The clinician must be familiar with the normal embryological, anatomical and biomechanical features of the hip joint. -

Morphological Variation of the Proximal Femur in Selected Skeletal Remains
MORPHOLOGICAL VARIATION OF THE PROXIMAL FEMUR IN SELECTED SKELETAL REMAINS A Thesis by Jessica Lynn Brown B.S. Michigan State University, 2002 Submitted to the College of Liberal Arts and Sciences and the faculty of the Graduate School of Wichita State University in partial fulfillment of the requirements for the degree of Master of Arts May 2006 MORPHOLOGICAL VARIATION OF THE PROXIMAL FEMUR IN SELECTED SKELETAL REMAINS I have examined the final copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Master of Arts with a major in Anthropology. __________________________________________ Peer H. Moore-Jansen, Ph.D., Committee Chair We have read this thesis and recommend its acceptance: __________________________________________ Robert Lawless, Ph.D., Committee Member __________________________________________ John Carter, Ph.D., Committee Member ii DEDICATION To My Family and Friends iii ACKNOWLEDGEMENTS Funding for this research came from three generous sources. Dr. Todd Fenton provided me with the opportunity to experience Albania’s past and present in two separate field seasons. The Moore-Jansen Scholarship and the Nancy Berner Fund through the Anthropology Department at Wichita State University provided financial support to study the Hamann-Todd collection in Cleveland. The expense of travel and accommodations during data collection during all of these experiences was greatly appreciated by these considerate awards. I would like to thank all the members of my thesis committee, Dr. Peer Moore-Jansen, Dr. Robert Lawless, and Dr. John Carter for their comments and suggestions. Throughout this process Dr. Peer Moore-Jansen provided invaluable guidance on how to conduct and report meaningful research, which will stay with me always. -
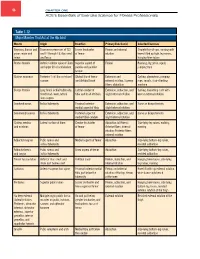
Table 1-12 Major Muscles That Act at the Hip Joint
46 Chapter one ACE’s Essentials of Exercise Science for Fitness Professionals Table 1-12 Major Muscles That Act at the Hip Joint Muscle Origin Insertion Primary Function(s) Selected Exercises Iliopsoas: Iliacus and Transverse processes of T12 Lesser trochanter Flexion and external Straight-leg sit-ups, running with psoas major and and L1 through L5; iliac crest of femur rotation knees lifted up high, leg raises, minor and fossa hanging knee raises Rectus femoris Anterior-inferior spine of ilium Superior aspect of Flexion Running, leg press, squat, and upper lift of acetabulum patella and patellar jumping rope tendon 1 Gluteus maximus Posterior /4 of iliac crest and Gluteal line of femur Extension and Cycling, plyometrics, jumping sacrum and iliotibial band external rotation; Superior rope, squats, stair-climbing fibers: abduction machine Biceps femoris Long head: ischial tuberosity; Lateral condyle of Extension, abduction, and Cycling, hamstring curls with Short head: lower, lateral tibia and head of fibula slight external rotation knee in external rotation linea aspera Semitendinosus Ischial tuberosity Proximal anterior- Extension, adduction, and Same as biceps femoris medial aspect of tibia slight internal rotation Semimembranosus Ischial tuberosity Posterior aspect of Extension, adduction, and Same as biceps femoris medial tibial condyle slight internal rotation Gluteus medius Lateral surface of ilium Greater trochanter Abduction (all fibers); Side-lying leg raises, walking, and minimus of femur Anterior fibers: internal running rotation; -

Macroanatomy of the Bones of Pelvis and Hind Limb of an Asian Elephant (Elephas Maximus)
Int. J. Morphol., 31(4):1473-1478, 2013. Macroanatomy of the Bones of Pelvis and Hind Limb of an Asian Elephant (Elephas maximus) Macroanatomía de los Huesos de la Pelvis y del Miembro Posterior de un Elefante Asiático (Elephas maximus) Subrata Kumar Shil; Md. Abul Quasem; Mohammad Lutfur Rahman; A. S. M. Golam Kibria; Mohi Uddin & A. S. M. Lutful Ahasan SHIL, S. K.; QUASEM, M. A.; RAHMAN, M. L.; KIBRIA, A. S. M. G.; UDDIN, M. & AHASAN, A. S. M. L. Macroanatomy of the bones of pelvis and hind limb of an Asian Elephant (Elephas maximus). Int. J. Morphol., 31(4):1473-1478, 2013. SUMMARY: Recent excavated skeleton of an adult female Asian Elephant (Elephas maximus), died in dystokia in Bangladesh was used for macro anatomical study. Some unique morphological features of bones of hind limb were observed. Pelvic canal was more oval and the wings of ilium were wider. Rump slope was about 36°. Angle between femur and tibia was close to 180°. In Femur, the major trochanter was located at the lower level of head. Minor trochanter, fovea capitis and trochanteric ridge were absent. Supracondyloid fossa was shallow but the intercondyloid fossa was deep. Posterior surface of patella possessed a blunt vertical ridge. The articular surfaces of both tibial condyles were clearly concave. The tibia and the fibula were articulated proximally and distally with keeping a wide interosseous space. Instead of tibial tuberosity, there was an elongated triangular depression in proximal part. There were six tarsal bones arranged in three rows. The comparative size of the distal tarsal bones were III+IV > I > II. -

DORSAL MUSCLES of the HINDLIMB (Ca)
Fascia thoracolumbalis Fascia glutea Fascia lata Lamina superficialis Lamina profunda Fascia cruris DORSAL MUSCLES OF THE HINDLIMB (ca) M. gluteus superficialis o Origin: sacrum and first caudal vertebrae, partly from sacrotuberous ligament; (and by means of deep gluteal fascia also from cranial dorsal iliac spine) o Insertion: on tuberositas glutea (below greater trochanter) o Action: extension of hip M. gluteus medius o Origin: crista iliaca and gluteal surface of iliac bone o Insertion: greater trochanter of femur o Action: strongest extensor of hip joint M. piriformis o Origin: last sacral and first caudal vertebrae o Insertion: greater trochanter of femur o Action: extension of hip joint M. gluteus profundus o Origin: gluteal surface and body of iliac bone o Insertion: greater trochanter of femur o Action: extension of hip joint Interspecies differences M. gluteus superficialis in bo, su: fused with m. biceps femoris and they form m. gluteobiceps, eq: inserts on trochanter tertius M. piriformis in eq, bo, su: fused with m. gluteus medius 1 2 DEEP MUSCLES OF THE HINDLIMB (ca) M. obturatorius externus o Origin: outer surface of pelvis, around foramen obturatum o Insertion: trochanteric fossa of femur o Action: lateral rotation (supination) of hindlimb M. quadratus femoris o Origin: ventral surface of tabula ossis ischii (medial to tuber ischiadicum) o Insertion: trochanteric fossa of femur o Action: extension of hip joint and lateral rotation of hindlimb M. obturatorius internus o Origin: inner surface of pelvis around for. obturatum (from regions of ramus cranialis et caudalis ossis pubis, ramus ossis ischii and tabula ossis ischii) o Insertion: after crossing lesser sciatic notch it will attach in trochanteric fossa of femur; its tendon runs over the muscle belly of m. -

Research Article Gross Anatomical Studies on the Hind Limb of the West African Giraffe (Giraffa Camelopardalis Peralta)
Hindawi Veterinary Medicine International Volume 2021, Article ID 8818525, 6 pages https://doi.org/10.1155/2021/8818525 Research Article Gross Anatomical Studies on the Hind Limb of the West African Giraffe (Giraffa camelopardalis peralta) Kenechukwu T. Onwuama , Sulaiman O. Salami, Esther S. Kigir, and Alhaji Z. Jaji Department of Veterinary Anatomy, University of Ilorin, Ilorin, Nigeria Correspondence should be addressed to Kenechukwu T. Onwuama; [email protected] Received 8 July 2020; Accepted 26 June 2021; Published 3 July 2021 Academic Editor: Antonio Ortega-Pacheco Copyright © 2021 Kenechukwu T. Onwuama et al. +is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. +is study on the gross anatomy of the West African giraffe’s hind limb was aimed at investigating the unique morphological features and number of bones making up this region of the skeleton. Two (2) adults obtained as carcasses at different times after postmortem examination were prepared to extract the bones via cold water maceration for use in the study. +e appearance of the ossa coxarum and its features presented similarities to that of the horse. However, differences were evident in the convex cranial border of the ilium, small less prominent coxal tuber, and wider interval between the opposite sacral tuber and an oval obturator foramen. Common features reported in most species such as the gluteal line and psoas tubercle were absent. +e long femur presented proximally; the greater trochanter connected obliquely via the trochanteric crest to the lesser trochanter. -

Lower Extremity Muscle Table
Robert Frysztak, PhD. Structure of the Human Body Loyola University Chicago Stritch School of Medicine LOWER EXTREMITY MUSCLE TABLE PROXIMAL ATTACHMENT DISTAL ATTACHMENT MUSCLE INNERVATION MAIN ACTIONS BLOOD SUPPLY MUSCLE GROUP (ORIGIN) (INSERTION) Lateral condyle of tibia, proximal 3/4 of Middle and distal phalanges of lateral Extends lateral four digits and Extensor digitorum longus anterior surface of interosseous Deep fibular nerve Anterior tibial artery Anterior leg four digits dorsiflexes foot at ankle membrane and fibula Middle part of anterior surface of fibula Dorsal aspect of base of distal phalanx Extends great toe, dorsiflexes foot at Extensor hallucis longus Deep fibular nerve Anterior tibial artery Anterior leg and interosseous membrane of great toe ankle Distal third of anterior surface of fibula Dorsiflexes foot at ankle and aids in Fibularis peroneus tertius Dorsum of base of 5th metatarsal Deep fibular nerve Anterior tibial artery Anterior leg and interosseous membrane eversion of foot Lateral condyle, proximal half of lateral Medial plantar surfaces of medial Dorsiflexes foot at ankle and inverts Tibialis anterior Deep fibular nerve Anterior tibial artery Anterior leg tibia, interosseous membrane cuneiform and base of 1st metatarsal foot Pulls suprapatellar bursa superiorly Articularis genus Distal femur on anterior surface Suprapatellar bursa Femoral nerve Femoral artery Anterior thigh with extension of knee First tendon into dorsal surface of base Aids the extensor digitorum longus in Superolateral surface of calcaneus, -

Osteology and Radiographic Anatomy of the Hind Limbs in Marshdeer (Blastocerus Dichotomus)1
Pesq. Vet. Bras. 35(12):997-1001, dezembro 2015 DOI: 10.1590/S0100-736X2015001200009 Osteology and radiographic anatomy of the hind limbs in Marshdeer (Blastocerus dichotomus)1 2 5 3 3 4 Bruno C. Schimming *, Sheila C. Rahal , Daniela A. Shigue 3, Juliana L. Linardi , Luiz ABSTRACT.- C. Vulcano and Carlos R. Teixeira Osteology and radiographic anatomy of the hind limbs in Marshdeer (Blas- tocerus dichotomus Schimming). Pesquisa B.C., Rahal Veterinária S.C., Shigue Brasileira D.A., Linardi35(12):997-1001 J.L., Vulcano. Departamento L.C. & Teixeira de C.R. 2015. [email protected] Anatomia, Universidade Estadual Paulista, Cx. Postal 510, Botucatu, SP 18618-970, Brazil. E-mail: The knowledge of anatomical structures found in wild animals is important for the practice of medical and surgicalBlastocerus clinic. Thus, dichotomus the aim of as this a reference study was for toclinical describe use andthe osteology and radiographic anatomy of the femur, patella, tibia, fibula, tarsal, metatarsal and phalanges of the Marshdeer species identification. Most structures were similar to those found in domestic animals, onewith described special features for the ofhorse. this species.B. dichotomus Noteworthy is, for example, the absence of the third trochanter of the femur. Although a ruminant, the Marshdeer has a fibuyla similar to the has four fingers on each limb, formed through three phalanges, only the third and fourth finger touch the ground, and the second and fifth finger is rudimentary. It has four proximal and two distal sesamoid bones, and sesamoid Blastocerus dichotomus, bones near the gastrocnemius muscle do not exist. RESUMO.- [AnatomiaINDEX TERMS:óssea e Marshdeer, radiográfica do membro thigh, leg, wild animals, anatomy. -

Oryctolagus Cuniculus
JOURNAL OF ADVANCED VETERINARY AND ANIMAL RESEARCH ISSN 2311-7710 (Electronic) http://doi.org/10.5455/javar.2018.e292 December 2018 A periodical of the Network for the Veterinarians of Bangladesh (BDvetNET) VOL 5, NO. 4, PAGES 410–419 ORIGINAL ARTICLE Comparative morphological interpretations on the bones of the pelvic limb of New Zealand rabbit (Oryctolagus cuniculus) and domestic cat Felis( domestica) Hanaa Mohamed El-Ghazali, Eman Ismail El-behery Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44511, Egypt ABSTRACT ARTICLE HISTORY Objective:Regarding the displaying of the main differences between the pelvic limb of rabbit and cat. Received June 06, 2018 Materials and methods: Our work was performed on 10 New Zealand rabbits (Oryctolagus cunic- Revised August 10, 2018 ulus) and domestic cats (Felis domestica) with variable ages and of both sexes. After weighing of Accepted August 12, 2018 the animals, sedation, and anesthesia, the animals were examined radiographically. The bones of Published November 09, 2018 the pelvic limb were prepared, measured for its length/cm then described and compared. KEYWORDS Results: The iliac tuberosity and the conversion of the acetabular notch into foramen were char- acteristics of Os coxae of the rabbit. The intertrochanteric crest was detected on the femur of the Acetabulum; fabellae; cat. In the rabbit, the leg interosseous space was located in the proximal third of this region while intertrochanteric crest; unguicular process in the cat, it was extended along its length. The first metatarsal was undeveloped in the cat but was absent in the rabbit so metatarsal were four in the rabbit and five in the cat. -

Gluteal Region
Gluteal region DR. GITANJALI KHORWAL Gluteal region • The transitional area between the trunk and the lower extremity. • The gluteal region includes the rounded, posterior buttocks and the laterally placed hip region. Bony framework L4 • S2 Greater sciatic foramen Lesser sciatic foramen Gluteal Aponeurosis • This is attached to the lateral border of the iliac crest superiorly, and • splits anteriorly to enclose tensor fasciae latae and posteriorly to enclose gluteus maximus. Muscles of Gluteal region Superficial Layer • Gluteus maximus • Tensor fasciae latae Muscles of Gluteal region Intermediate layer • Gluteus medius • Piriformis • Superior gemellus. • Tendon of obturator internus. • Inferior gemellus • Quadratus femoris • Upper part of Adductor magnus • And Hamstrings Muscles of Gluteal region Deep layer • Gluteus minimus • Reflected head of rectus femoris • Tendinous insertion of obturator externus Gluteus Maximus Origins: posterior end of the iliac crest, posterior surface of the sacrum, coccyx and sacrotuberous ligament. Insertions: ilio-tibial tract( 3/4)and gluteal tuberosity.(1/4 ) Innervation: inferior gluteal nerve - [ Ventral rami of L5, S1,2] - emerges below the piriformis muscle to penetrate the deep surface of the gluteus maximus with accompanying vessels. Actions • Extensor at hip joint during running and climbing upstairs. • Chief antigravity muscle in the standing up from a seated position. • Strong lateral rotation of the thigh. Its upper fibres are active in powerful abduction of the thigh. • It is a tensor of the fascia lata, and through the iliotibial tract it stabilizes the femur on the tibia when the extensor muscles of the knee are relaxed. Tensor Fascia Lata Small muscle close to the anterior border of the gluteus medius, at the dorsal surface of the ASIS.