Adrenoreceptor Antagonistsin Essential Tremor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Optum Essential Health Benefits Enhanced Formulary PDL January
PENICILLINS ketorolac tromethamineQL GENERIC mefenamic acid amoxicillin/clavulanate potassium nabumetone amoxicillin/clavulanate potassium ER naproxen January 2016 ampicillin naproxen sodium ampicillin sodium naproxen sodium CR ESSENTIAL HEALTH BENEFITS ampicillin-sulbactam naproxen sodium ER ENHANCED PREFERRED DRUG LIST nafcillin sodium naproxen DR The Optum Preferred Drug List is a guide identifying oxacillin sodium oxaprozin preferred brand-name medicines within select penicillin G potassium piroxicam therapeutic categories. The Preferred Drug List may piperacillin sodium/ tazobactam sulindac not include all drugs covered by your prescription sodium tolmetin sodium drug benefit. Generic medicines are available within many of the therapeutic categories listed, in addition piperacillin sodium/tazobactam Fenoprofen Calcium sodium to categories not listed, and should be considered Meclofenamate Sodium piperacillin/tazobactam as the first line of prescribing. Tolmetin Sodium Amoxicillin/Clavulanate Potassium LOW COST GENERIC PREFERRED For benefit coverage or restrictions please check indomethacin your benefit plan document(s). This listing is revised Augmentin meloxicam periodically as new drugs and new prescribing LOW COST GENERIC naproxen kit information becomes available. It is recommended amoxicillin that you bring this list of medications when you or a dicloxacillin sodium CARDIOVASCULAR covered family member sees a physician or other penicillin v potassium ACE-INHIBITORS healthcare provider. GENERIC QUINOLONES captopril ANTI-INFECTIVES -

Management of Chronic Problems
MANAGEMENT OF CHRONIC PROBLEMS INTERACTIONS BETWEEN ALCOHOL AND DRUGS A. Leary,* T. MacDonald† SUMMARY concerned. Alcohol may alter the effects of the drug; drug In western society alcohol consumption is common as is may change the effects of alcohol; or both may occur. the use of therapeutic drugs. It is not surprising therefore The interaction between alcohol and drug may be that concomitant use of these should occur frequently. The pharmacokinetic, with altered absorption, metabolism or consequences of this combination vary with the dose of elimination of the drug, alcohol or both.2 Alcohol may drug, the amount of alcohol taken, the mode of affect drug pharmacokinetics by altering gastric emptying administration and the pharmacological effects of the drug or liver metabolism. Drugs may affect alcohol kinetics by concerned. Interactions may be pharmacokinetic or altering gastric emptying or inhibiting gastric alcohol pharmacodynamic, and while coincidental use of alcohol dehydrogenase (ADH).3 This may lead to altered tissue may affect the metabolism or action of a drug, a drug may concentrations of one or both agents, with resultant toxicity. equally affect the metabolism or action of alcohol. Alcohol- The results of concomitant use may also be principally drug interactions may differ with acute and chronic alcohol pharmacodynamic, with combined alcohol and drug effects ingestion, particularly where toxicity is due to a metabolite occurring at the receptor level without important changes rather than the parent drug. There is both inter- and intra- in plasma concentration of either. Some interactions have individual variation in the response to concomitant drug both kinetic and dynamic components and, where this is and alcohol use. -

Supplementary Materials
Supplementary Materials Table S1. The significant drug pairs in potential DDIs examined by the two databases. Micromedex Drugs.com List of drugs paired PK-PD Mechanism details 1. Amiodarone— PD Additive QT-interval prolongation Dronedarone 2. Amiodarone— PK CYP3A inhibition by Ketoconazole Ketoconazole 3. Ciprofloxacin— PD Additive QT-interval prolongation Dronedarone 4. Cyclosporine— PK CYP3A inhibition by Cyclosporine Dronedarone 5. Dronedarone— PK CYP3A inhibition by Erythromycin Erythromycin 6. Dronedarone— PD Additive QT-interval prolongation Flecainide 7. Dronedarone— PK CYP3A4 inhibition by Itraconazole Itraconazole 8. Dronedarone— PK Contraindication Major CYP3A inhibition by Ketoconazole Ketoconazole 9. Dronedarone— PD Additive QT-interval prolongation Procainamide PD 10. Dronedarone—Sotalol Additive QT-interval prolongation 11. Felodipine— PK CYP3A inhibition by Itraconazole Itraconazole 12. Felodipine— PK CYP3A inhibition by Ketoconazole Ketoconazole 13. Itraconazole— PK CYP3A inhibition by Itraconazole Nisoldipine 14. Ketoconazole— PK CYP3A inhibition by Ketoconazole Nisoldipine 15. Praziquantel— PK CYP induction by Rifampin Rifampin PD 1. Amikacin—Furosemide Additive or synergistic toxicity 2. Aminophylline— Decreased clearance of PK Ciprofloxacin Theophylline by Ciprofloxacin 3. Aminophylline— PK Decreased hepatic metabolism Mexiletine 4. Amiodarone— PD Additive effects on QT interval Ciprofloxacin 5. Amiodarone—Digoxin PK P-glycoprotein inhibition by Amiodarone 6. Amiodarone— PD, PK Major Major Additive effects on QT Erythromycin prolongation, CYP3A inhibition by Erythromycin 7. Amiodarone— PD, PK Flecainide Antiarrhythmic inhibition by Amiodarone, CYP2D inhibition by Amiodarone 8. Amiodarone— PK CYP3A inhibition by Itraconazole Itraconazole 9. Amiodarone— PD Antiarrhythmic inhibition by Procainamide Amiodarone 10. Amiodarone— PK CYP induction by Rifampin Rifampin PD Additive effects on refractory 11. Amiodarone—Sotalol potential 12. Amiodarone— PK CYP3A inhibition by Verapamil Verapamil 13. -

Australian Adverse Drug Reactions Bulletin, Volume 24 No 6
Prepared by the Adverse Drug Reactions Advisory Committee (ADRAC). Members of ADRAC are Associate Professor Duncan Topliss (Chair), Dr Vicki Kotsirilos, Professor David Isaacs, Dr Cecilie Lander, Professor John McNeil, Associate Professor Peter Pillans, Dr Simone Strasser, Dr Dana Wainwright AUSTRALIAN ADVERSE DRUG REACTIONS BULLETIN Volume 24, Number 6, December 2005 ! Medicines and QT prolongation ! Skin reactions with glucosamine ! Warfarin-induced skin necrosis Please report all suspected reactions to these Drugs of Current Interest Aripiprazole (Abilify) Levetiracetam (Keppra) Atomoxetine (Strattera) Pimecrolimus (Elidel) Ezetimibe (Ezetrol) Pregabalin (Lyrica) Fenofibrate (Lipidil) Reboxetine (Edronax) Iron sucrose (Venofer) Teriparatide (Fortéo) 1. Medicines and QT prolongation In the past decade, a number of medicines have Other, non-drug factors which may be associated been either withdrawn from the market or had their with QT prolongation include female sex, use restricted because of QT interval advanced age, bradycardia, cardiac failure, cardiac prolongation.1 The QT interval is considered to be ischaemia and electrolyte disturbances. prolonged if it is more than 450 msec after adjusting for heart rate (‘corrected QT interval’). Drug interactions are an important cause of QT prolongation and torsade de pointes, even in Drug-induced QT prolongation may be caused by healthy people with no risk factors. These blockade of cardiac potassium channels, and can interactions are of two types. The first involves the lead to a life-threatening polymorphic ventricular combined use of two or more drugs, each with a tachycardia known as torsade de pointes. As of QT prolonging effect, i.e. the simultaneous use of June 2005, ADRAC had received 140 reports of two drugs from the Table. -

Guideline for Preoperative Medication Management
Guideline: Preoperative Medication Management Guideline for Preoperative Medication Management Purpose of Guideline: To provide guidance to physicians, advanced practice providers (APPs), pharmacists, and nurses regarding medication management in the preoperative setting. Background: Appropriate perioperative medication management is essential to ensure positive surgical outcomes and prevent medication misadventures.1 Results from a prospective analysis of 1,025 patients admitted to a general surgical unit concluded that patients on at least one medication for a chronic disease are 2.7 times more likely to experience surgical complications compared with those not taking any medications. As the aging population requires more medication use and the availability of various nonprescription medications continues to increase, so does the risk of polypharmacy and the need for perioperative medication guidance.2 There are no well-designed trials to support evidence-based recommendations for perioperative medication management; however, general principles and best practice approaches are available. General considerations for perioperative medication management include a thorough medication history, understanding of the medication pharmacokinetics and potential for withdrawal symptoms, understanding the risks associated with the surgical procedure and the risks of medication discontinuation based on the intended indication. Clinical judgement must be exercised, especially if medication pharmacokinetics are not predictable or there are significant risks associated with inappropriate medication withdrawal (eg, tolerance) or continuation (eg, postsurgical infection).2 Clinical Assessment: Prior to instructing the patient on preoperative medication management, completion of a thorough medication history is recommended – including all information on prescription medications, over-the-counter medications, “as needed” medications, vitamins, supplements, and herbal medications. Allergies should also be verified and documented. -
Keeping up with the Pace of Antiarrhythmic Drugs ANNMARIE PALATNIK, APN,BC, MSN Coordinator of Continuing Education • Virtua Health • Marlton, N.J
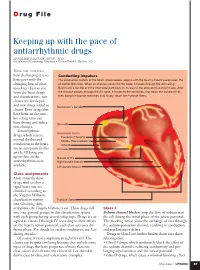
D rug File Keeping up with the pace of antiarrhythmic drugs ANNMARIE PALATNIK, APN,BC, MSN Coordinator of Continuing Education • Virtua Health • Marlton, N.J. HAVE YOU NOTICED how challenging it is to Conducting impulses keep pace with the The conduction system of the heart, shown below, begins with the heart’s natural pacemaker, the changing beat of phar- sinoatrial (SA) node. When an impulse leaves the SA node, it travels through the atria along macology? Just as you Bachmann’s bundle and the internodal pathways on its way to the atrioventricular (AV) node. After learn the latest drugs the impulse passes through the AV node, it travels to the ventricles, first down the bundle of His, and classifications, new then along the bundle branches and, finally, down the Purkinje fibers. classes are developed and new drugs added to Bachmann’s bundle classes. Even drugs that have been on the mar- ket a long time can have dosing and indica- SA node tion changes. Antiarrhythmic Internodal tracts drugs, which restore Posterior (Thorel’s) normal rhythm and Middle (Wenckebach’s) conduction to the heart, Anterior are no exception. In this AV node article, I’ll bring you up-to-date on the Bundle of His antiarrhythmics now Right bundle branch available. Left bundle branch Class assignments Most antiarrhythmic drugs used to slow a rapid heart rate are classified according to the Vaughn Williams classification system Purkinje fibers (see Classifying Anti- arrhythmics the Vaughn Williams way). These drugs fall Class I into four general groups in this classification system Sodium channel blockers stop the flow of sodium into with each group having several subgroups. -
Clomipramine 25 Mg Capsules, Hard
Package leaflet: Information for the patient Other medicines and Clomipramine Tell your doctor if you are taking, have recently Clomipramine 10 mg Capsules, Hard taken or might take any other medicines. Clomipramine 25 mg Capsules, Hard Some medicines may increase the side effects of Clomipramine 50 mg Capsules, Hard Clomipramine and may sometimes cause very clomipramine hydrochloride serious reactions. Do not take any other medicines whilst taking Clomipramine without first talking to Read all of this leaflet carefully before you your doctor, especially: start taking this medicine because it contains - medicines for depression, particularly MAOIs (see important information for you. section “Do not take” above) e.g. tranylcypromine, - Keep this leaflet. You may need to read it again. phenelzine, moclobemide; SSRIs e.g. fluoxetine (or - If you have any further questions, ask your have taken within the last 3 weeks), fluvoxamine, doctor or pharmacist. paroxetine, sertraline; SNaRIs e.g. venlafaxine; - This medicine has been prescribed for you only. tricyclic and tetracyclic antidepressants e.g. Do not pass it on to others. It may harm them, amitriptyline, dothiepin, maprotiline even if their signs of illness are the same as yours. - diuretics, also known as ‘water tablets’, e.g. - If you get any side effects, talk to your doctor bendroflumethiazide, furosemide or pharmacist. This includes any possible side - anaesthetics, used for the temporary loss of effects not listed in this leaflet. See section 4. bodily sensation - antihistamines e.g. terfenadine What is in this leaflet - medicines for other mental health conditions 1. What Clomipramine is and what it is used for. such as schizophrenia or manic depression 2. -

Oral Antiarrhythmic Drugs in Converting Recent Onset Atrial Fibrillation
Review article Oral antiarrhythmic drugs in converting recent onset atrial fibrillation • Vera H.M. Deneer, Marieke B.I. Borgh, J. Herre Kingma, Loraine Lie-A-Huen and Jacobus R.B.J. Brouwers Introduction Pharm World Sci 2004; 26: 66–78. © 2004 Kluwer Academic Publishers. Printed in the Netherlands. Atrial fibrillation is the most common arrhythmia. The incidence of atrial fibrillation depends on the age of V.H.M. Deneer (correspondence, e-mail: the study population. The incidence varies between 2 [email protected]), M.B.I. Borgh, L. Lie-A-Huen: Department of Clinical Pharmacy or 3 new cases per 1,000 population per year between J.H. Kingma: Department of Cardiology, St Antonius Hospital, the ages of 55 and 64 years to 35 new cases per 1,000 Koekoekslaan 1, 3435 CM Nieuwegein, The Netherlands population per year between the ages of 85 and 94 J.R.B.J. Brouwers: Groningen University Institute for Drug 1 Exploration (GUIDE), Section of Pharmacotherapy, University years . Treatment of an episode of paroxysmal atrial fi- of Groningen, Antonius Deusinglaan 1, 9713 AV Groningen, brillation consists of restoring sinus rhythm by DC- The Netherlands electrical cardioversion or by the intravenous adminis- Key words tration of an antiarrhythmic drug, but frequently the Atrial fibrillation arrhythmia spontaneously terminates1–3. After one or Amiodarone more episodes of atrial fibrillation chronic prophylac- Antiarrhythmic drugs Digoxin tic treatment with an antiarrhythmic drug is often Episodic treatment started for maintenance of sinus rhythm4–8. Another Flecainide treatment strategy consists of allowing the arrhythmia Propafenone Quinidine to exist in combination with pharmacological ven- Sotalol tricular rate control. -

Sotalol Considerations For
Sotalol (Betapace, Sorine) Considerations for Use* US/FDA Approved Indications: Heart Rhythm Control for Atrial Fibrillation Black Box Warning* Patients should be in a facility for at least 3 days with ECG monitoring, cardiac resuscitation available, and CrCl calculated when initiating or re-initiating. Do not substitute Betapace for Betapace AF. Abrupt cessation may exacerbate angina pectoris and MI. Mechanism of Action Prolongs cardiac repolarization (Class III antiarrhythmic properties). Dosing† Maintenance: 80 to 320 mg PO every 12 hrs, based on QTC interval and renal function Hepatic Impairment: No adjustments needed Renal Impairment: CrCl 40-60 mL/min: dose every 24 hours CrCl < 40 mL/min: contraindicated Contraindications Asthma AV block Bradycardia decompensated heart failure hypokalemia pulmonary edema QT prolongation renal failure sick sinus syndrome Major Side Effects torsades de pointes, HF, bradycardia Dosage forms and Strengths PO: 80, 120, 160, 240 mg tablets IV: 150mg/10ml solution for injection Special Notes Non-selective beta-blocker. Avoid in patients with asthma. Potassium and magnesium levels should be within normal range prior to initiating and during therapy. To minimize the risk of induced arrhythmia, patients initiated or re-initiated on sotalol should be placed for a minimum of 3 days (on their maintenance dose) in a facility that can provide cardiac resuscitation and continuous electrocardiographic monitoring. Monitor serum creatinine, magnesium, potassium, heart rate, blood pressure; EKG. Has many drug interactions. Abrupt cessation my precipitate angina, MI, arrhythmias, or rebound HTN; discontinue by tapering over 1-2 weeks. Do not substitute Betapace® for Betapace AF® Counseling Do not abruptly discontinue without physician’s advice. -

Information for the User Amitriptyline Hydrochloride 25Mg/5Ml Oral Solution Amitriptyline Hydrochloride 50Mg/5Ml Oral Solution
Package leaflet: Information for the user Amitriptyline Hydrochloride 25mg/5ml oral solution Amitriptyline Hydrochloride 50mg/5ml oral solution Read all of this leaflet carefully before you start to take this medicine because it contains important information for you. - Keep this leaflet. You may need to read it again. - If you have any further questions, please ask your doctor or pharmacist. - This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. - If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet 1. What Amitriptyline Oral Solution is and what it is used for 2. What you need to know before you take Amitriptyline Oral Solution 3. How to take Amitriptyline Oral Solution 4. Possible side effects 5. How to store Amitriptyline Oral Solution 6. Contents of the pack and other information 1. What Amitriptyline Oral Solution is and what it is used for Amitriptyline Oral Solution belongs to a group of medicines known as tricyclic antidepressants. This medicine is used to treat: • Depression in adults (major depressive episodes) • Neuropathic pain in adults • Chronic tension type headache prophylaxis in adults • Migraine prophylaxis in adults • Bed-wetting at night in children aged 6 years and above, only when organic causes, such as spina bifida and related disorders, have been excluded and no response has been achieved to all other non-drug and drug treatments, including muscle relaxants and desmopressin. -

Sotalol | Memorial Sloan Kettering Cancer Center
PATIENT & CAREGIVER EDUCATION Sotalol This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: US Betapace; Betapace AF; Sorine; Sotylize Brand Names: Canada APO-Sotalol; CO Sotalol [DSC]; DOM-Sotalol; JAMP-Sotalol; MED Sotalol; PMS- Sotalol; PRO-Sotalol [DSC]; RATIO-Sotalol; RIVA-Sotalol Warning This drug may cause a life-threatening type of heartbeat that is not normal (prolonged QT interval). Talk with your doctor if you have a long QT on ECG. You will have to start and restart this drug in a setting where your heart will be watched nonstop. Talk with your doctor. If you have kidney disease, talk to your doctor. What is this drug used for? It is used to treat certain types of life-threatening abnormal heartbeats. It is used to keep a normal heartbeat in people who have a certain type of abnormal heartbeat (atrial fibrillation or atrial flutter). Sotalol 1/8 What do I need to tell my doctor BEFORE I take this drug? If you are allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had. If you have any of these health problems: Asthma or other lung or breathing problems that cause shortness of breath or wheezing, heart failure (weak heart), certain types of abnormal heartbeats called heart block or sick sinus syndrome, or a slow heartbeat. If you have any of these health problems: Low potassium or magnesium levels. -

List of Formulary Drug Removals
July 2021 Formulary Drug Removals Below is a list of medicines by drug class that have been removed from your plan’s formulary. If you continue using one of the drugs listed below and identified as a Formulary Drug Removal, you may be required to pay the full cost. If you are currently using one of the formulary drug removals, ask your doctor to choose one of the generic or brand formulary options listed below. Category Formulary Drug Formulary Options Drug Class Removals Acromegaly SANDOSTATIN LAR SOMATULINE DEPOT SIGNIFOR LAR SOMAVERT Allergies dexchlorpheniramine levocetirizine Antihistamines Diphen Elixir RyClora CARBINOXAMINE TABLET 6 MG Allergies BECONASE AQ flunisolide spray, fluticasone spray, mometasone spray, DYMISTA Nasal Steroids / Combinations OMNARIS QNASL ZETONNA Anticonvulsants topiramate ext-rel capsule carbamazepine, carbamazepine ext-rel, clobazam, divalproex sodium, (generics for QUDEXY XR only) divalproex sodium ext-rel, gabapentin, lamotrigine, lamotrigine ext-rel, levetiracetam, levetiracetam ext-rel, oxcarbazepine, phenobarbital, phenytoin, phenytoin sodium extended, primidone, rufinamide, tiagabine, topiramate, valproic acid, zonisamide, FYCOMPA, OXTELLAR XR, TROKENDI XR, VIMPAT, XCOPRI BANZEL SUSPENSION clobazam, lamotrigine, rufinamide, topiramate, TROKENDI XR ONFI SABRIL vigabatrin ZONEGRAN carbamazepine, carbamazepine ext-rel, divalproex sodium, divalproex sodium ext-rel, gabapentin, lamotrigine, lamotrigine ext-rel, levetiracetam, levetiracetam ext-rel, oxcarbazepine, phenobarbital, phenytoin, phenytoin sodium