Oral Antiarrhythmic Drugs in Converting Recent Onset Atrial Fibrillation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table 2. 2012 AGS Beers Criteria for Potentially
Table 2. 2012 AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults Strength of Organ System/ Recommendat Quality of Recomm Therapeutic Category/Drug(s) Rationale ion Evidence endation References Anticholinergics (excludes TCAs) First-generation antihistamines Highly anticholinergic; Avoid Hydroxyzin Strong Agostini 2001 (as single agent or as part of clearance reduced with e and Boustani 2007 combination products) advanced age, and promethazi Guaiana 2010 Brompheniramine tolerance develops ne: high; Han 2001 Carbinoxamine when used as hypnotic; All others: Rudolph 2008 Chlorpheniramine increased risk of moderate Clemastine confusion, dry mouth, Cyproheptadine constipation, and other Dexbrompheniramine anticholinergic Dexchlorpheniramine effects/toxicity. Diphenhydramine (oral) Doxylamine Use of diphenhydramine in Hydroxyzine special situations such Promethazine as acute treatment of Triprolidine severe allergic reaction may be appropriate. Antiparkinson agents Not recommended for Avoid Moderate Strong Rudolph 2008 Benztropine (oral) prevention of Trihexyphenidyl extrapyramidal symptoms with antipsychotics; more effective agents available for treatment of Parkinson disease. Antispasmodics Highly anticholinergic, Avoid Moderate Strong Lechevallier- Belladonna alkaloids uncertain except in Michel 2005 Clidinium-chlordiazepoxide effectiveness. short-term Rudolph 2008 Dicyclomine palliative Hyoscyamine care to Propantheline decrease Scopolamine oral secretions. Antithrombotics Dipyridamole, oral short-acting* May -

Optum Essential Health Benefits Enhanced Formulary PDL January
PENICILLINS ketorolac tromethamineQL GENERIC mefenamic acid amoxicillin/clavulanate potassium nabumetone amoxicillin/clavulanate potassium ER naproxen January 2016 ampicillin naproxen sodium ampicillin sodium naproxen sodium CR ESSENTIAL HEALTH BENEFITS ampicillin-sulbactam naproxen sodium ER ENHANCED PREFERRED DRUG LIST nafcillin sodium naproxen DR The Optum Preferred Drug List is a guide identifying oxacillin sodium oxaprozin preferred brand-name medicines within select penicillin G potassium piroxicam therapeutic categories. The Preferred Drug List may piperacillin sodium/ tazobactam sulindac not include all drugs covered by your prescription sodium tolmetin sodium drug benefit. Generic medicines are available within many of the therapeutic categories listed, in addition piperacillin sodium/tazobactam Fenoprofen Calcium sodium to categories not listed, and should be considered Meclofenamate Sodium piperacillin/tazobactam as the first line of prescribing. Tolmetin Sodium Amoxicillin/Clavulanate Potassium LOW COST GENERIC PREFERRED For benefit coverage or restrictions please check indomethacin your benefit plan document(s). This listing is revised Augmentin meloxicam periodically as new drugs and new prescribing LOW COST GENERIC naproxen kit information becomes available. It is recommended amoxicillin that you bring this list of medications when you or a dicloxacillin sodium CARDIOVASCULAR covered family member sees a physician or other penicillin v potassium ACE-INHIBITORS healthcare provider. GENERIC QUINOLONES captopril ANTI-INFECTIVES -

Management of Chronic Problems
MANAGEMENT OF CHRONIC PROBLEMS INTERACTIONS BETWEEN ALCOHOL AND DRUGS A. Leary,* T. MacDonald† SUMMARY concerned. Alcohol may alter the effects of the drug; drug In western society alcohol consumption is common as is may change the effects of alcohol; or both may occur. the use of therapeutic drugs. It is not surprising therefore The interaction between alcohol and drug may be that concomitant use of these should occur frequently. The pharmacokinetic, with altered absorption, metabolism or consequences of this combination vary with the dose of elimination of the drug, alcohol or both.2 Alcohol may drug, the amount of alcohol taken, the mode of affect drug pharmacokinetics by altering gastric emptying administration and the pharmacological effects of the drug or liver metabolism. Drugs may affect alcohol kinetics by concerned. Interactions may be pharmacokinetic or altering gastric emptying or inhibiting gastric alcohol pharmacodynamic, and while coincidental use of alcohol dehydrogenase (ADH).3 This may lead to altered tissue may affect the metabolism or action of a drug, a drug may concentrations of one or both agents, with resultant toxicity. equally affect the metabolism or action of alcohol. Alcohol- The results of concomitant use may also be principally drug interactions may differ with acute and chronic alcohol pharmacodynamic, with combined alcohol and drug effects ingestion, particularly where toxicity is due to a metabolite occurring at the receptor level without important changes rather than the parent drug. There is both inter- and intra- in plasma concentration of either. Some interactions have individual variation in the response to concomitant drug both kinetic and dynamic components and, where this is and alcohol use. -

Supplementary Materials
Supplementary Materials Table S1. The significant drug pairs in potential DDIs examined by the two databases. Micromedex Drugs.com List of drugs paired PK-PD Mechanism details 1. Amiodarone— PD Additive QT-interval prolongation Dronedarone 2. Amiodarone— PK CYP3A inhibition by Ketoconazole Ketoconazole 3. Ciprofloxacin— PD Additive QT-interval prolongation Dronedarone 4. Cyclosporine— PK CYP3A inhibition by Cyclosporine Dronedarone 5. Dronedarone— PK CYP3A inhibition by Erythromycin Erythromycin 6. Dronedarone— PD Additive QT-interval prolongation Flecainide 7. Dronedarone— PK CYP3A4 inhibition by Itraconazole Itraconazole 8. Dronedarone— PK Contraindication Major CYP3A inhibition by Ketoconazole Ketoconazole 9. Dronedarone— PD Additive QT-interval prolongation Procainamide PD 10. Dronedarone—Sotalol Additive QT-interval prolongation 11. Felodipine— PK CYP3A inhibition by Itraconazole Itraconazole 12. Felodipine— PK CYP3A inhibition by Ketoconazole Ketoconazole 13. Itraconazole— PK CYP3A inhibition by Itraconazole Nisoldipine 14. Ketoconazole— PK CYP3A inhibition by Ketoconazole Nisoldipine 15. Praziquantel— PK CYP induction by Rifampin Rifampin PD 1. Amikacin—Furosemide Additive or synergistic toxicity 2. Aminophylline— Decreased clearance of PK Ciprofloxacin Theophylline by Ciprofloxacin 3. Aminophylline— PK Decreased hepatic metabolism Mexiletine 4. Amiodarone— PD Additive effects on QT interval Ciprofloxacin 5. Amiodarone—Digoxin PK P-glycoprotein inhibition by Amiodarone 6. Amiodarone— PD, PK Major Major Additive effects on QT Erythromycin prolongation, CYP3A inhibition by Erythromycin 7. Amiodarone— PD, PK Flecainide Antiarrhythmic inhibition by Amiodarone, CYP2D inhibition by Amiodarone 8. Amiodarone— PK CYP3A inhibition by Itraconazole Itraconazole 9. Amiodarone— PD Antiarrhythmic inhibition by Procainamide Amiodarone 10. Amiodarone— PK CYP induction by Rifampin Rifampin PD Additive effects on refractory 11. Amiodarone—Sotalol potential 12. Amiodarone— PK CYP3A inhibition by Verapamil Verapamil 13. -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

The Elimination of High Doses of Phenytoin In
THE ELIMINATION OF HIGH DOSES OF PHENYTOIN IN MAN AND THE RABBIT by Peter Chinwah Department of Clinical Pharmacology St. Vincent's Hospital Darlinghurst, 2010, N.S.W. AUSTRALIA A Thesis submitted for the Degree of Master of Science in the University of New South Wales February, 1987 Ul·lilJ:2?.S1TY QF N.S.W. ~ 14 JUN 1988 f L!.:.": --":c_A:_'''"lARY I (i) ACKNOWLEDGEMENTS I wish to thank Professor Denis Wade for the opportunity to undertake this thesis in the Department of Clinical Pharmacology and for his overseeing and supervision of this project. I would like to express my gratitude to Dr Garry Graham, Senior Lecturer in the Department of Physiology and Pharmacology at the University of New South Wales, for his significant contribution in discussion and critical review of this thesis. I also wish to thank my colleagues of the Department of Clinical Pharmacology and Toxicology at St Vincent's Hospital for their assistance during the course of the project. Finally, I am grateful to Dr Ken Williams of the Department of Clinical Pharmacology and Toxicology at St Vincent's Hospital for many hours of discussion, and his admonishment, long suffering and perserverence which have made this thesis possible. (ii) ABSTRACT The pharmacokinetics of phenytoin were studied in twelve patients who presented to casualty with phenytoin toxicity. These patients were divided into 3 groups according to the type of plasma elimination profiles observed. Group 1. consisted of 3 patients who demonstrated first order elimination kinetics with long elimination half lives (70-106 hours). Group 2. consisted of 6 patients who showed saturable elimination kinetics (mean estimated terminal half life 28 hours). -

Antiparasitic Properties of Cardiovascular Agents Against Human Intravascular Parasite Schistosoma Mansoni
pharmaceuticals Article Antiparasitic Properties of Cardiovascular Agents against Human Intravascular Parasite Schistosoma mansoni Raquel Porto 1, Ana C. Mengarda 1, Rayssa A. Cajas 1, Maria C. Salvadori 2 , Fernanda S. Teixeira 2 , Daniel D. R. Arcanjo 3 , Abolghasem Siyadatpanah 4, Maria de Lourdes Pereira 5 , Polrat Wilairatana 6,* and Josué de Moraes 1,* 1 Research Center for Neglected Diseases, Guarulhos University, Praça Tereza Cristina 229, São Paulo 07023-070, SP, Brazil; [email protected] (R.P.); [email protected] (A.C.M.); [email protected] (R.A.C.) 2 Institute of Physics, University of São Paulo, São Paulo 05508-060, SP, Brazil; [email protected] (M.C.S.); [email protected] (F.S.T.) 3 Department of Biophysics and Physiology, Federal University of Piaui, Teresina 64049-550, PI, Brazil; [email protected] 4 Ferdows School of Paramedical and Health, Birjand University of Medical Sciences, Birjand 9717853577, Iran; [email protected] 5 CICECO-Aveiro Institute of Materials & Department of Medical Sciences, University of Aveiro, 3810-193 Aveiro, Portugal; [email protected] 6 Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University, Bangkok 10400, Thailand * Correspondence: [email protected] (P.W.); [email protected] (J.d.M.) Citation: Porto, R.; Mengarda, A.C.; Abstract: The intravascular parasitic worm Schistosoma mansoni is a causative agent of schistosomiasis, Cajas, R.A.; Salvadori, M.C.; Teixeira, a disease of great global public health significance. Praziquantel is the only drug available to F.S.; Arcanjo, D.D.R.; Siyadatpanah, treat schistosomiasis and there is an urgent demand for new anthelmintic agents. -

Australian Adverse Drug Reactions Bulletin, Volume 24 No 6
Prepared by the Adverse Drug Reactions Advisory Committee (ADRAC). Members of ADRAC are Associate Professor Duncan Topliss (Chair), Dr Vicki Kotsirilos, Professor David Isaacs, Dr Cecilie Lander, Professor John McNeil, Associate Professor Peter Pillans, Dr Simone Strasser, Dr Dana Wainwright AUSTRALIAN ADVERSE DRUG REACTIONS BULLETIN Volume 24, Number 6, December 2005 ! Medicines and QT prolongation ! Skin reactions with glucosamine ! Warfarin-induced skin necrosis Please report all suspected reactions to these Drugs of Current Interest Aripiprazole (Abilify) Levetiracetam (Keppra) Atomoxetine (Strattera) Pimecrolimus (Elidel) Ezetimibe (Ezetrol) Pregabalin (Lyrica) Fenofibrate (Lipidil) Reboxetine (Edronax) Iron sucrose (Venofer) Teriparatide (Fortéo) 1. Medicines and QT prolongation In the past decade, a number of medicines have Other, non-drug factors which may be associated been either withdrawn from the market or had their with QT prolongation include female sex, use restricted because of QT interval advanced age, bradycardia, cardiac failure, cardiac prolongation.1 The QT interval is considered to be ischaemia and electrolyte disturbances. prolonged if it is more than 450 msec after adjusting for heart rate (‘corrected QT interval’). Drug interactions are an important cause of QT prolongation and torsade de pointes, even in Drug-induced QT prolongation may be caused by healthy people with no risk factors. These blockade of cardiac potassium channels, and can interactions are of two types. The first involves the lead to a life-threatening polymorphic ventricular combined use of two or more drugs, each with a tachycardia known as torsade de pointes. As of QT prolonging effect, i.e. the simultaneous use of June 2005, ADRAC had received 140 reports of two drugs from the Table. -
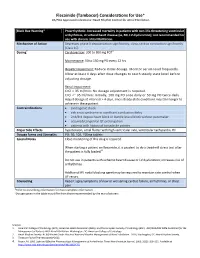
Flecainide Considerations For
Flecainide (Tambocor) Considerations for Use* US/FDA Approved Indications: Heart Rhythm Control for Atrial Fibrillation Black Box Warning* Proarrhythmic. Increased mortality in patients with non-life-threatening ventricular arrhythmias, structural heart disease (ie, MI, LV dysfunction); not recommended for use with chronic atrial fibrillation. Mechanism of Action Depresses phase 0 depolarization significantly, slows cardiac conduction significantly (Class 1C). Dosing† Cardioversion: 200 to 300 mg PO‡1 Maintenance: 50 to 150 mg PO every 12 hrs Hepatic Impairment: Reduce initial dosage. Monitor serum level frequently. Allow at least 4 days after dose changes to reach steady state level before adjusting dosage. Renal Impairment: CrCl > 35 ml/min: No dosage adjustment is required. CrCl <= 35 ml/min: Initially, 100 mg PO once daily or 50 mg PO twice daily. Adjust dosage at intervals > 4 days, since steady-state conditions may take longer to achieve in these patient Contraindications cardiogenic shock sick sinus syndrome or significant conduction delay 2nd/3rd degree heart block or bundle brand block without pacemaker acquired/congenital QT prolongation patients with history of torsade de pointes Major Side Effects hypotension, atrial flutter with high ventricular rate, ventricular tachycardia, HF Dosage forms and Strengths PO: 50, 100, 150mg tablets Special Notes Close monitoring of this drug is required. When starting a patient on flecainide, it is prudent to do a treadmill stress test after the patient is fully loaded.4 Do not use in patients with ischemic heart disease or LV dysfunction; increases risk of arrhythmias. Additional AV nodal blocking agent may be required to maintain rate control when AF recurs. -

Multaq, INN-Dronedarone
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT MULTAQ 400 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 400 mg of dronedarone (as hydrochloride). Excipient with known effect: Each tablet also contains 41.65 mg of lactose (as monohydrate). For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Film-coated tablet (tablet). White, oblong shaped tablets, engraved with a double wave marking on one side and “4142”code on the other side. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications MULTAQ is indicated for the maintenance of sinus rhythm after successful cardioversion in adult clinically stable patients with paroxysmal or persistent atrial fibrillation (AF). Due to its safety profile (see sections 4.3 and 4.4), MULTAQ should only be prescribed after alternative treatment options have been considered. MULTAQ must not be given to patients with left ventricular systolic dysfunction or to patients with current or previous episodes of heart failure. 4.2 Posology and method of administration Treatment should be initiated and monitored only under specialist supervision (see section 4.4). Treatment with dronedarone can be initiated in an outpatient setting. Treatment with Class I or III antiarrhythmics (such as flecainide, propafenone, quinidine, disopyramide, dofetilide, sotalol, amiodarone) must be stopped before starting dronedarone. There is limited information on the optimal timing to switch from amiodarone to dronedarone. It should be considered that amiodarone may have a long duration of action after discontinuation due to its long half-life. If a switch is envisaged, this should be done under the supervision of a specialist (see sections 4.3 and 5.1). -

A Proposal for the Clinical Use of Flecainide
A Proposal for the Clinical Use of Flecainide JEFFREY L. ANDERSON, MD, JAMES R. STEWART, MD, and BARRY J. CREVEY, MD Effective antiarrhythmic therapy requires a carefully sponse rate is observed in preventing induction of considered approach, including an understanding sustained ventricular tachycardia, and these pa- of the arrhythmia, the underlying cardiac disease tients should be carefully selected. Flecainide is and the drug’s pharmacokinetics. Flecainide is a promising in the treatment of supraventricular new antiarrhythmic drug that may soon be released tachycardias using atrioventricular nodal or extra- for general use. Flecainide demonstrates unsur- nodal reentrant pathways, although this use is still passed efficacy in chronic ventricular arrhythmias investigational in the United States. The drug’s use in stable patients and may become a first-choice for arrhythmias during acute myocardial infarction drug because of its ease of administration, efficacy requires further study. Flecainide possesses modest and favorable tolerance. Twice-daily dosing with negative inotropic potential. Proarrhythmic or other 100 to 200 mg usually provides effective therapy. adverse reactions have occurred primarily in set- Clinical experience suggests flecainide to be indi- tings of high drug level, poor ventricular function cated in the treatment of uniform and multiform or refractory, malignant arrhythmias, suggesting ventricular premature complexes, coupled ven- caution in these groups. tricular premature complexes, and episodes of nonsustained -

Guideline for Preoperative Medication Management
Guideline: Preoperative Medication Management Guideline for Preoperative Medication Management Purpose of Guideline: To provide guidance to physicians, advanced practice providers (APPs), pharmacists, and nurses regarding medication management in the preoperative setting. Background: Appropriate perioperative medication management is essential to ensure positive surgical outcomes and prevent medication misadventures.1 Results from a prospective analysis of 1,025 patients admitted to a general surgical unit concluded that patients on at least one medication for a chronic disease are 2.7 times more likely to experience surgical complications compared with those not taking any medications. As the aging population requires more medication use and the availability of various nonprescription medications continues to increase, so does the risk of polypharmacy and the need for perioperative medication guidance.2 There are no well-designed trials to support evidence-based recommendations for perioperative medication management; however, general principles and best practice approaches are available. General considerations for perioperative medication management include a thorough medication history, understanding of the medication pharmacokinetics and potential for withdrawal symptoms, understanding the risks associated with the surgical procedure and the risks of medication discontinuation based on the intended indication. Clinical judgement must be exercised, especially if medication pharmacokinetics are not predictable or there are significant risks associated with inappropriate medication withdrawal (eg, tolerance) or continuation (eg, postsurgical infection).2 Clinical Assessment: Prior to instructing the patient on preoperative medication management, completion of a thorough medication history is recommended – including all information on prescription medications, over-the-counter medications, “as needed” medications, vitamins, supplements, and herbal medications. Allergies should also be verified and documented.