The Atmospheric Steam Engine As Energy Converter for Low and Medium Temperature Thermal Energy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Overview of Materials Used for the Basic Elements of Hydraulic Actuators and Sealing Systems and Their Surfaces Modification Methods
materials Review Overview of Materials Used for the Basic Elements of Hydraulic Actuators and Sealing Systems and Their Surfaces Modification Methods Justyna Skowro ´nska* , Andrzej Kosucki and Łukasz Stawi ´nski Institute of Machine Tools and Production Engineering, Lodz University of Technology, ul. Stefanowskiego 1/15, 90-924 Lodz, Poland; [email protected] (A.K.); [email protected] (Ł.S.) * Correspondence: [email protected] Abstract: The article is an overview of various materials used in power hydraulics for basic hydraulic actuators components such as cylinders, cylinder caps, pistons, piston rods, glands, and sealing systems. The aim of this review is to systematize the state of the art in the field of materials and surface modification methods used in the production of actuators. The paper discusses the requirements for the elements of actuators and analyzes the existing literature in terms of appearing failures and damages. The most frequently applied materials used in power hydraulics are described, and various surface modifications of the discussed elements, which are aimed at improving the operating parameters of actuators, are presented. The most frequently used materials for actuators elements are iron alloys. However, due to rising ecological requirements, there is a tendency to looking for modern replacements to obtain the same or even better mechanical or tribological parameters. Sealing systems are manufactured mainly from thermoplastic or elastomeric polymers, which are characterized by Citation: Skowro´nska,J.; Kosucki, low friction and ensure the best possible interaction of seals with the cooperating element. In the A.; Stawi´nski,Ł. Overview of field of surface modification, among others, the issue of chromium plating of piston rods has been Materials Used for the Basic Elements discussed, which, due, to the toxicity of hexavalent chromium, should be replaced by other methods of Hydraulic Actuators and Sealing of improving surface properties. -

Isobaric Expansion Engines: New Opportunities in Energy Conversion for Heat Engines, Pumps and Compressors
energies Concept Paper Isobaric Expansion Engines: New Opportunities in Energy Conversion for Heat Engines, Pumps and Compressors Maxim Glushenkov 1, Alexander Kronberg 1,*, Torben Knoke 2 and Eugeny Y. Kenig 2,3 1 Encontech B.V. ET/TE, P.O. Box 217, 7500 AE Enschede, The Netherlands; [email protected] 2 Chair of Fluid Process Engineering, Paderborn University, Pohlweg 55, 33098 Paderborn, Germany; [email protected] (T.K.); [email protected] (E.Y.K.) 3 Chair of Thermodynamics and Heat Engines, Gubkin Russian State University of Oil and Gas, Leninsky Prospekt 65, Moscow 119991, Russia * Correspondence: [email protected] or [email protected]; Tel.: +31-53-489-1088 Received: 12 December 2017; Accepted: 4 January 2018; Published: 8 January 2018 Abstract: Isobaric expansion (IE) engines are a very uncommon type of heat-to-mechanical-power converters, radically different from all well-known heat engines. Useful work is extracted during an isobaric expansion process, i.e., without a polytropic gas/vapour expansion accompanied by a pressure decrease typical of state-of-the-art piston engines, turbines, etc. This distinctive feature permits isobaric expansion machines to serve as very simple and inexpensive heat-driven pumps and compressors as well as heat-to-shaft-power converters with desired speed/torque. Commercial application of such machines, however, is scarce, mainly due to a low efficiency. This article aims to revive the long-known concept by proposing important modifications to make IE machines competitive and cost-effective alternatives to state-of-the-art heat conversion technologies. Experimental and theoretical results supporting the isobaric expansion technology are presented and promising potential applications, including emerging power generation methods, are discussed. -

RECIPROCATING ENGINES Franck Nicolleau
RECIPROCATING ENGINES Franck Nicolleau To cite this version: Franck Nicolleau. RECIPROCATING ENGINES. Master. RECIPROCATING ENGINES, Sheffield, United Kingdom. 2010, pp.189. cel-01548212 HAL Id: cel-01548212 https://hal.archives-ouvertes.fr/cel-01548212 Submitted on 27 Jun 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial| 4.0 International License Mechanical Engineering - 14 May 2010 -1- UNIVERSITY OF SHEFFIELD Department of Mechanical Engineering Mappin street, Sheffield, S1 3JD, England RECIPROCATING ENGINES Autumn Semester 2010 MEC403 - MEng, semester 7 - MEC6403 - MSc(Res) Dr. F. C. G. A. Nicolleau MD54 Telephone: +44 (0)114 22 27700. Direct Line: +44 (0)114 22 27867 Fax: +44 (0)114 22 27890 email: F.Nicolleau@sheffield.ac.uk http://www.shef.ac.uk/mecheng/mecheng cms/staff/fcgan/ MEng 4th year Course Tutor : Pr N. Qin European and Year Abroad Tutor : C. Pinna MSc(Res) and MPhil Course Director : F. C. G. A. Nicolleau c 2010 F C G A Nicolleau, The University of Sheffield -2- Combustion engines Table of content -3- Table of content Table of content 3 Nomenclature 9 Introduction 13 Acknowledgement 16 I - Introduction and Fundamentals of combustion 17 1 Introduction to combustion engines 19 1.1 Pistonengines.................................. -

Advanced Power Cycles with Mixtures As the Working Fluid
Advanced Power Cycles with Mixtures as the Working Fluid Maria Jonsson Doctoral Thesis Department of Chemical Engineering and Technology, Energy Processes Royal Institute of Technology Stockholm, Sweden, 2003 Advanced Power Cycles with Mixtures as the Working Fluid Maria Jonsson Doctoral Thesis Department of Chemical Engineering and Technology, Energy Processes Royal Institute of Technology Stockholm, Sweden, 2003 TRITA-KET R173 ISSN 1104-3466 ISRN KTH/KET/R--173--SE ISBN 91-7283-443-9 Contact information: Royal Institute of Technology Department of Chemical Engineering and Technology, Division of Energy Processes SE-100 44 Stockholm Sweden Copyright © Maria Jonsson, 2003 All rights reserved Printed in Sweden Universitetsservice US AB Stockholm, 2003 Advanced Power Cycles with Mixtures as the Working Fluid Maria Jonsson Department of Chemical Engineering and Technology, Energy Processes Royal Institute of Technology, Stockholm, Sweden Abstract The world demand for electrical power increases continuously, requiring efficient and low-cost methods for power generation. This thesis investigates two advanced power cycles with mixtures as the working fluid: the Kalina cycle, alternatively called the ammonia-water cycle, and the evaporative gas turbine cycle. These cycles have the potential of improved performance regarding electrical efficiency, specific power output, specific investment cost and cost of electricity compared with the conventional technology, since the mixture working fluids enable efficient energy recovery. This thesis shows that the ammonia-water cycle has a better thermodynamic performance than the steam Rankine cycle as a bottoming process for natural gas- fired gas and gas-diesel engines, since the majority of the ammonia-water cycle configurations investigated generated more power than steam cycles. -

Dynamic and Thermodynamic Examination of a Two- Stroke Internal Combustion Engine
Politeknik Dergisi, 2016; 19 (2) : 141-154 Journal of Polytechnic, 2016; 19 (2) : 141-154 Dynamic and Thermodynamic Examination of a Two- Stroke Internal Combustion Engine Duygu İPCİ*, Halit KARABULUTa aGazi University Technology Faculty Automotive Engineering Department (Geliş / Received : 27.06.2015 ; Kabul / Accepted : 24.07.2015) ABSTRACT In this study the combined dynamic and thermodynamic analysis of a two-stroke internal combustion engine was carried out. The variation of the heat, given to the working fluid during the heating process of the thermodynamic cycle, was modeled with the Gaussian function. The dynamic model of the piston driving mechanism was established by means of nine equations, five of them are motion equations and four of them are kinematic relations. Equations are solved by using a numerical method based on the Taylor series. By means of introducing practical specific values, the dynamic and thermodynamic behaviors of the engine were examined. Variations of several engine performance parameters with engine speed and charging pressure were examined. The brake thermal efficiency, cooling loss, friction loss and exhaust loss of the engine were predicted as about 37%, 28%, 4%, and 31% respectively for 3000 rpm engine speed and 1 bar charging pressure. Speed fluctuation was found to be 3% for 3000 rpm engine speed and 45 Nm torque. Keywords: Two stroke engine, thermodynamic analysis, dynamic analysis, power and torque estimation, speed fluctuations. ÖZ Bu araştırmada iki zamanlı bir içten yanmalı motorun dinamik ve termodinamik birleşik analizi yapılmıştır. Termodinamik çevrimin yanma süresince çalışma akışkanına verilen ısının değişimi Gauss fonksiyonu ile modellenmiştir. Piston hareket mekanizmasının dinamik modeli dokuz denklemle modellenmiş olup bunlardan beşi hareket denklemi, dördü kinematik ilişkidir. -

THESIS WASTE HEAT RECOVERY from a HIGH TEMPERATURE DIESEL ENGINE Submitted by Jonas E. Adler Department of Mechanical Engineerin
THESIS WASTE HEAT RECOVERY FROM A HIGH TEMPERATURE DIESEL ENGINE Submitted by Jonas E. Adler Department of Mechanical Engineering In partial fulfillment of the requirements For the Degree of Master of Science Colorado State University Fort Collins, Colorado Fall 2017 Master’s Committee: Advisor: Todd M. Bandhauer Daniel B. Olsen Sybil E. Sharvelle Copyright by Jonas E. Adler 2017 All Rights Reserved ABSTRACT WASTE HEAT RECOVERY FROM A HIGH TEMPERATURE DIESEL ENGINE Government-mandated improvements in fuel economy and emissions from internal combustion engines (ICEs) are driving innovation in engine efficiency. Though incremental efficiency gains have been achieved, most combustion engines are still only 30-40% efficient at best, with most of the remaining fuel energy being rejected to the environment as waste heat through engine coolant and exhaust gases. Attempts have been made to harness this waste heat and use it to drive a Rankine cycle and produce additional work to improve efficiency. Research on waste heat recovery (WHR) demonstrates that it is possible to improve overall efficiency by converting wasted heat into usable work, but relative gains in overall efficiency are typically minimal (~5-8%) and often do not justify the cost and space requirements of a WHR system. The primary limitation of the current state-of-the-art in WHR is the low temperature of the engine coolant (~90°C), which minimizes the WHR from a heat source that represents between 20% and 30% of the fuel energy. The current research proposes increasing the engine coolant temperature to improve the utilization of coolant waste heat as one possible path to achieving greater WHR system effectiveness. -

25 Kw Low-Temperature Stirling Engine for Heat Recovery, Solar, and Biomass Applications
25 kW Low-Temperature Stirling Engine for Heat Recovery, Solar, and Biomass Applications Lee SMITHa, Brian NUELa, Samuel P WEAVERa,*, Stefan BERKOWERa, Samuel C WEAVERb, Bill GROSSc aCool Energy, Inc, 5541 Central Avenue, Boulder CO 80301 bProton Power, Inc, 487 Sam Rayburn Parkway, Lenoir City TN 37771 cIdealab, 130 W. Union St, Pasadena CA 91103 *Corresponding author: [email protected] Keywords: Stirling engine, waste heat recovery, concentrating solar power, biomass power generation, low-temperature power generation, distributed generation ABSTRACT This paper covers the design, performance optimization, build, and test of a 25 kW Stirling engine that has demonstrated > 60% of the Carnot limit for thermal to electrical conversion efficiency at test conditions of 329 °C hot side temperature and 19 °C rejection temperature. These results were enabled by an engine design and construction that has minimal pressure drop in the gas flow path, thermal conduction losses that are limited by design, and which employs a novel rotary drive mechanism. Features of this engine design include high-surface- area heat exchangers, nitrogen as the working fluid, a single-acting alpha configuration, and a design target for operation between 150 °C and 400 °C. 1 1. INTRODUCTION Since 2006, Cool Energy, Inc. (CEI) has designed, fabricated, and tested five generations of low-temperature (150 °C to 400 °C) Stirling engines that drive internally integrated electric alternators. The fifth generation of engine built by Cool Energy is rated at 25 kW of electrical power output, and is trade-named the ThermoHeart® Engine. Sources of low-to-medium temperature thermal energy, such as internal combustion engine exhaust, industrial waste heat, flared gas, and small-scale solar heat, have relatively few methods available for conversion into more valuable electrical energy, and the thermal energy is usually wasted. -

Systematic Methods for Working Fluid Selection and the Design, Integration and Control of Organic Rankine Cycles—A Review
Energies 2015, 8, 4755-4801; doi:10.3390/en8064755 OPEN ACCESS energies ISSN 1996-1073 www.mdpi.com/journal/energies Review Systematic Methods for Working Fluid Selection and the Design, Integration and Control of Organic Rankine Cycles—A Review Patrick Linke 1,*, Athanasios I. Papadopoulos 2,† and Panos Seferlis 3,† 1 Department of Chemical Engineering, Texas A&M University at Qatar, P.O. Box 23874, Education City, 77874 Doha, Qatar 2 Chemical Process and Energy Resources Institute, Centre for Research and Technology-Hellas, Thermi, 57001 Thessaloniki, Greece; E-Mail: [email protected] 3 Department of Mechanical Engineering, Aristotle University of Thessaloniki, P.O. Box 484, 54124 Thessaloniki, Greece; E-Mail: [email protected] † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +974-4423-0251; Fax: +974-4423-0011. Academic Editor: Roberto Capata Received: 1 March 2015 / Accepted: 15 May 2015 / Published: 26 May 2015 Abstract: Efficient power generation from low to medium grade heat is an important challenge to be addressed to ensure a sustainable energy future. Organic Rankine Cycles (ORCs) constitute an important enabling technology and their research and development has emerged as a very active research field over the past decade. Particular focus areas include working fluid selection and cycle design to achieve efficient heat to power conversions for diverse hot fluid streams associated with geothermal, solar or waste heat sources. Recently, a number of approaches have been developed that address the systematic selection of efficient working fluids as well as the design, integration and control of ORCs. -

I Iiti I ,T Ti Ti
1 NASA-TM-86875 NASA TM-86875 19?tJCJOI/S-8S I iiti I ti I lii ,t ti i I LI LANGLEY RESEAHCH CENTER .. L.IBRARY, NASA VIHGINIA Lanny Thieme .. N ional Aeronautics and Space Administration Lewis Research Center 1 Prepared for 111111111111111111111111111111111111111111111 NF00096 DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. Printed in the United States of America Available from National Technical Information Service U.S. Department of Commerce 5285 Port Royal Road Springfield, VA 22161 NTIS price codes1 Printed copy:A02 Microfiche copy: A01 1Codes are used for pricing all publications. The code is determined by the number of pages in the publication. Information pertaining to the pricing codes can be found in the current issues of the following publications, which are generally available in most libraries: Energy Research Abstracts (ERA); Government Reports Announcements and Index (GRA and I); Scientific and Technical Abstract Reports (STAR); and publication, NTIS-PR-360 available from NTIS at the above address. -
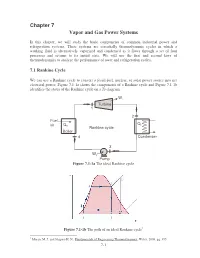
Chapter 7 Vapor and Gas Power Systems
Chapter 7 Vapor and Gas Power Systems In this chapter, we will study the basic components of common industrial power and refrigeration systems. These systems are essentially thermodyanmic cycles in which a working fluid is alternatively vaporized and condensed as it flows through a set of four processes and returns to its initial state. We will use the first and second laws of thermodynamics to analyze the performance of ower and refrigeration cycles. 7.1 Rankine Cycle We can use a Rankine cycle to convert a fossil-fuel, nuclear, or solar power source into net electrical power. Figure 7.1-1a shows the components of a Rankine cycle and Figure 7.1-1b identifies the states of the Rankine cycle on a Ts diagram. Wt Turbine 1 2 Fuel QC air QH Rankine cycle Boiler 4 Condenser 3 Wp Pump Figure 7.1-1a The ideal Rankine cycle Figure 7.1-1b The path of an ideal Rankine cycle1 1 Moran, M. J. and Shapiro H. N., Fundamentals of Engineering Thermodynamics, Wiley, 2008, pg. 395 7-1 In the ideal Rankine cycle, the working fluid undergoes four reversible processes: Process 1-2: Isentropic expansion of the working fluid from the turbine from saturated vapor at state 1 or superheated vapor at state 1’ to the condenser pressure. Process 2-3: Heat transfer from the working fluid as it flows at constant pressure through the condenser with saturated liquid at state 3. Process 3-4: Isentropic compression in the pump to state 4 in the compressed liquid region. Process 4-1: Heat transfer to the working fluid as it flows at constant pressure through the boiler to complete the cycle. -

Air Standard Assumptions Some Definitions for Reciprocation Engines
Air Standard Assumptions In power engines, energy is provided by burning fuel within the system boundaries, i.e., internal combustion engines. The following assumptions are commonly known as the air- standard assumptions: 1- The working fluid is air, which continuously circulates in a closed loop (cycle). Air is considered as ideal gas. 2- All the processes in (ideal) power cycles are internally reversible. 3- Combustion process is modeled by a heat-addition process from an external source. 4- The exhaust process is modeled by a heat-rejection process that restores the working fluid (air) at its initial state. Assuming constant specific heats, (@25°C) for air, is called cold-air-standard assumption. Some Definitions for Reciprocation Engines: The reciprocation engine is one the most common machines that is being used in a wide variety of applications from automobiles to aircrafts to ships, etc. Intake Exhaust valve valve TDC Bore Stroke BDC Fig. 3-1: Reciprocation engine. Top dead center (TDC): The position of the piston when it forms the smallest volume in the cylinder. Bottom dead center (BDC): The position of the piston when it forms the largest volume in the cylinder. Stroke: The largest distance that piston travels in one direction. Bore: The diameter of the piston. M. Bahrami ENSC 461 (S 11) IC Engines 1 Clearance volume: The minimum volume formed in the cylinder when the piston is at TDC. Displacement volume: The volume displaced by the piston as it moves between the TDC and BDC. Compression ratio: The ratio of maximum to minimum (clearance) volumes in the cylinder: V V r max BDC Vmin VTDC Mean effective pressure (MEP): A fictitious (constant throughout the cycle) pressure that if acted on the piston will produce the work. -

External Combustion Engines: Prospects for Vehicular Application
EXTERNAL COMBUSTION ENGINES: PROSPECTS FOR VEHICULAR APPLICATION Roy A. Renner, California steam Bus Project, International Research and Technology Corporation, San Ramon, California External combustion engines are discussed as possible alternatives to the internal combustion engine for vehicle propulsion. Potential advantages are low levels of exhaust pollution, quiet operation, high starting torque, and possible lower costs during a vehicle lifetime. Present experience with the California steam Bus Project indicates that competitive road per formance is obtainable with steam-powered city buses, but fuel consump tion is higher than with a diesel engine. Opportunities remain open for the evolutionary improvement of thermal efficiency. Logical early applica tions include stop-and-go fleet vehicles. •GASOLINE and diesel engines have been preferred prime movers for motor vehicles during a period of many years. Despite the almost universal application of the internal combusion engine (ICE), the criteria for vehicular power plants are now being seriously reexamined. Alternatives to the ICE are being reconsidered in a new light (1, 2). The external combusion engine (ECE), of which the steam engine is the best known 0 xample, has been in use for more than 200 years. steam power was popular for auto- _obiles at the turn of this century. Prior to 1910, it was considered to be superior to the ICE for automobile propulsion in every way except first cost and convenience. Even then, the steam car was noted for its quietness and clean exhaust; freedom from gear shifting was also a decided advantage. Now that air pollution, noise, and congestion are factors no longer to be ignored, both the role of the vehicle and its source of power are being evaluated anew.