Foundations of Quantum Mechanics Lecture Notes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unit 1 Old Quantum Theory
UNIT 1 OLD QUANTUM THEORY Structure Introduction Objectives li;,:overy of Sub-atomic Particles Earlier Atom Models Light as clectromagnetic Wave Failures of Classical Physics Black Body Radiation '1 Heat Capacity Variation Photoelectric Effect Atomic Spectra Planck's Quantum Theory, Black Body ~diation. and Heat Capacity Variation Einstein's Theory of Photoelectric Effect Bohr Atom Model Calculation of Radius of Orbits Energy of an Electron in an Orbit Atomic Spectra and Bohr's Theory Critical Analysis of Bohr's Theory Refinements in the Atomic Spectra The61-y Summary Terminal Questions Answers 1.1 INTRODUCTION The ideas of classical mechanics developed by Galileo, Kepler and Newton, when applied to atomic and molecular systems were found to be inadequate. Need was felt for a theory to describe, correlate and predict the behaviour of the sub-atomic particles. The quantum theory, proposed by Max Planck and applied by Einstein and Bohr to explain different aspects of behaviour of matter, is an important milestone in the formulation of the modern concept of atom. In this unit, we will study how black body radiation, heat capacity variation, photoelectric effect and atomic spectra of hydrogen can be explained on the basis of theories proposed by Max Planck, Einstein and Bohr. They based their theories on the postulate that all interactions between matter and radiation occur in terms of definite packets of energy, known as quanta. Their ideas, when extended further, led to the evolution of wave mechanics, which shows the dual nature of matter -
![Arxiv:1206.1084V3 [Quant-Ph] 3 May 2019](https://docslib.b-cdn.net/cover/2699/arxiv-1206-1084v3-quant-ph-3-may-2019-82699.webp)
Arxiv:1206.1084V3 [Quant-Ph] 3 May 2019
Overview of Bohmian Mechanics Xavier Oriolsa and Jordi Mompartb∗ aDepartament d'Enginyeria Electr`onica, Universitat Aut`onomade Barcelona, 08193, Bellaterra, SPAIN bDepartament de F´ısica, Universitat Aut`onomade Barcelona, 08193 Bellaterra, SPAIN This chapter provides a fully comprehensive overview of the Bohmian formulation of quantum phenomena. It starts with a historical review of the difficulties found by Louis de Broglie, David Bohm and John Bell to convince the scientific community about the validity and utility of Bohmian mechanics. Then, a formal explanation of Bohmian mechanics for non-relativistic single-particle quantum systems is presented. The generalization to many-particle systems, where correlations play an important role, is also explained. After that, the measurement process in Bohmian mechanics is discussed. It is emphasized that Bohmian mechanics exactly reproduces the mean value and temporal and spatial correlations obtained from the standard, i.e., `orthodox', formulation. The ontological characteristics of the Bohmian theory provide a description of measurements in a natural way, without the need of introducing stochastic operators for the wavefunction collapse. Several solved problems are presented at the end of the chapter giving additional mathematical support to some particular issues. A detailed description of computational algorithms to obtain Bohmian trajectories from the numerical solution of the Schr¨odingeror the Hamilton{Jacobi equations are presented in an appendix. The motivation of this chapter is twofold. -

Particle-On-A-Ring” Suppose a Diatomic Molecule Rotates in Such a Way That the Vibration of the Bond Is Unaffected by the Rotation
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015 Lecture 17 2/25/15 Recommended Reading Atkins & DePaula 9.5 A. Background in Rotational Mechanics Two masses m1 and m2 connected by a weightless rigid “bond” of length r rotates with angular velocity rv where v is the linear velocity. As with vibrations we can render this rotation motion in simpler form by fixing one end of the bond to the origin terminating the other end with reduced mass , and allowing the reduced mass to rotate freely For two masses m1 and m2 separated by r the center of mass (CoM) is defined by the condition mr11 mr 2 2 (17.1) where m2,1 rr1,2 mm12 (17.2) Figure 17.1: Location of center of mass (CoM0 for two masses separated by a distance r. The moment of inertia I plays the role in the rotational energy that is played by mass in translational energy. The moment of inertia is 22 I mr11 mr 2 2 (17.3) Putting 17.3 into 17.4 we obtain I r 2 (17.4) mm where 12 . mm12 We can express the rotational kinetic energy K in terms of the angular momentum. The angular momentum is defined in terms of the cross product between the position vector r and the linear momentum vector p: Lrp (17.5) prprvrsin where the angle between p and r is ninety degrees for circular motion. As a cross product, the angular momentum vector L is perpendicular to the plane defined by the r and p vectors as shown in Figure 17.2 We can use equation 17.6 to obtain an expression for the kinetic energy in terms of the angular momentum L: Figure 17.2: The angular momentum L is a cross product of the position r vector and the linear momentum p=mv vector. -

Walther Nernst, Albert Einstein, Otto Stern, Adriaan Fokker
Walther Nernst, Albert Einstein, Otto Stern, Adriaan Fokker, and the Rotational Specific Heat of Hydrogen Clayton Gearhart St. John’s University (Minnesota) Max-Planck-Institut für Wissenschaftsgeschichte Berlin July 2007 1 Rotational Specific Heat of Hydrogen: Widely investigated in the old quantum theory Nernst Lorentz Eucken Einstein Ehrenfest Bohr Planck Reiche Kemble Tolman Schrödinger Van Vleck Some of the more prominent physicists and physical chemists who worked on the specific heat of hydrogen through the mid-1920s. See “The Rotational Specific Heat of Molecular Hydrogen in the Old Quantum Theory” http://faculty.csbsju.edu/cgearhart/pubs/sel_pubs.htm (slide show) 2 Rigid Rotator (Rotating dumbbell) •The rigid rotator was among the earliest problems taken up in the old (pre-1925) quantum theory. • Applications: Molecular spectra, and rotational contri- bution to the specific heat of molecular hydrogen • The problem should have been simple Molecular • relatively uncomplicated theory spectra • only one adjustable parameter (moment of inertia) • Nevertheless, no satisfactory theoretical description of the specific heat of hydrogen emerged in the old quantum theory Our story begins with 3 Nernst’s Heat Theorem Walther Nernst 1864 – 1941 • physical chemist • studied with Boltzmann • 1889: lecturer, then professor at Göttingen • 1905: professor at Berlin Nernst formulated his heat theorem (Third Law) in 1906, shortly after appointment as professor in Berlin. 4 Nernst’s Heat Theorem and Quantum Theory • Initially, had nothing to do with quantum theory. • Understand the equilibrium point of chemical reactions. • Nernst’s theorem had implications for specific heats at low temperatures. • 1906–1910: Nernst and his students undertook extensive measurements of specific heats of solids at low (down to liquid hydrogen) temperatures. -

Feynman Quantization
3 FEYNMAN QUANTIZATION An introduction to path-integral techniques Introduction. By Richard Feynman (–), who—after a distinguished undergraduate career at MIT—had come in as a graduate student to Princeton, was deeply involved in a collaborative effort with John Wheeler (his thesis advisor) to shake the foundations of field theory. Though motivated by problems fundamental to quantum field theory, as it was then conceived, their work was entirely classical,1 and it advanced ideas so radicalas to resist all then-existing quantization techniques:2 new insight into the quantization process itself appeared to be called for. So it was that (at a beer party) Feynman asked Herbert Jehle (formerly a student of Schr¨odinger in Berlin, now a visitor at Princeton) whether he had ever encountered a quantum mechanical application of the “Principle of Least Action.” Jehle directed Feynman’s attention to an obscure paper by P. A. M. Dirac3 and to a brief passage in §32 of Dirac’s Principles of Quantum Mechanics 1 John Archibald Wheeler & Richard Phillips Feynman, “Interaction with the absorber as the mechanism of radiation,” Reviews of Modern Physics 17, 157 (1945); “Classical electrodynamics in terms of direct interparticle action,” Reviews of Modern Physics 21, 425 (1949). Those were (respectively) Part III and Part II of a projected series of papers, the other parts of which were never published. 2 See page 128 in J. Gleick, Genius: The Life & Science of Richard Feynman () for a popular account of the historical circumstances. 3 “The Lagrangian in quantum mechanics,” Physicalische Zeitschrift der Sowjetunion 3, 64 (1933). The paper is reprinted in J. -

Aromaticity As a Guiding Concept for Spectroscopic Features and Nonlinear Optical Properties of Porphyrinoids
molecules Article Aromaticity as a Guiding Concept for Spectroscopic Features and Nonlinear Optical Properties of Porphyrinoids Tatiana Woller 1, Paul Geerlings 1, Frank De Proft 1, Benoît Champagne 2 ID and Mercedes Alonso 1,* ID 1 Eenheid Algemene Chemie (ALGC), Vrije Universiteit Brussel (VUB), Pleinlaan 2, 1050 Brussels, Belgium; [email protected] (T.W.); [email protected] (P.G.); [email protected] (F.D.P.) 2 Laboratoire de Chimie Théorique, Unité de Chimie Physique Théorique et Structurale, University of Namur, Rue de Bruxelles 61, B-5000 Namur, Belgium; [email protected] * Correspondence: [email protected] Academic Editors: Luis R. Domingo and Miquel Solà Received: 5 March 2018; Accepted: 24 May 2018; Published: 1 June 2018 Abstract: With their versatile molecular topology and aromaticity, porphyrinoid systems combine remarkable chemistry with interesting photophysical properties and nonlinear optical properties. Hence, the field of application of porphyrinoids is very broad ranging from near-infrared dyes to opto-electronic materials. From previous experimental studies, aromaticity emerges as an important concept in determining the photophysical properties and two-photon absorption cross sections of porphyrinoids. Despite a considerable number of studies on porphyrinoids, few investigate the relationship between aromaticity, UV/vis absorption spectra and nonlinear properties. To assess such structure-property relationships, we performed a computational study focusing on a series of Hückel porphyrinoids to: (i) assess their (anti)aromatic character; (ii) determine the fingerprints of aromaticity on the UV/vis spectra; (iii) evaluate the role of aromaticity on the NLO properties. Using an extensive set of aromaticity descriptors based on energetic, magnetic, structural, reactivity and electronic criteria, the aromaticity of [4n+2] π-electron porphyrinoids was evidenced as was the antiaromaticity for [4n] π-electron systems. -

The Concept of the Photon—Revisited
The concept of the photon—revisited Ashok Muthukrishnan,1 Marlan O. Scully,1,2 and M. Suhail Zubairy1,3 1Institute for Quantum Studies and Department of Physics, Texas A&M University, College Station, TX 77843 2Departments of Chemistry and Aerospace and Mechanical Engineering, Princeton University, Princeton, NJ 08544 3Department of Electronics, Quaid-i-Azam University, Islamabad, Pakistan The photon concept is one of the most debated issues in the history of physical science. Some thirty years ago, we published an article in Physics Today entitled “The Concept of the Photon,”1 in which we described the “photon” as a classical electromagnetic field plus the fluctuations associated with the vacuum. However, subsequent developments required us to envision the photon as an intrinsically quantum mechanical entity, whose basic physics is much deeper than can be explained by the simple ‘classical wave plus vacuum fluctuations’ picture. These ideas and the extensions of our conceptual understanding are discussed in detail in our recent quantum optics book.2 In this article we revisit the photon concept based on examples from these sources and more. © 2003 Optical Society of America OCIS codes: 270.0270, 260.0260. he “photon” is a quintessentially twentieth-century con- on are vacuum fluctuations (as in our earlier article1), and as- Tcept, intimately tied to the birth of quantum mechanics pects of many-particle correlations (as in our recent book2). and quantum electrodynamics. However, the root of the idea Examples of the first are spontaneous emission, Lamb shift, may be said to be much older, as old as the historical debate and the scattering of atoms off the vacuum field at the en- on the nature of light itself – whether it is a wave or a particle trance to a micromaser. -

Theory and Experiment in the Quantum-Relativity Revolution
Theory and Experiment in the Quantum-Relativity Revolution expanded version of lecture presented at American Physical Society meeting, 2/14/10 (Abraham Pais History of Physics Prize for 2009) by Stephen G. Brush* Abstract Does new scientific knowledge come from theory (whose predictions are confirmed by experiment) or from experiment (whose results are explained by theory)? Either can happen, depending on whether theory is ahead of experiment or experiment is ahead of theory at a particular time. In the first case, new theoretical hypotheses are made and their predictions are tested by experiments. But even when the predictions are successful, we can’t be sure that some other hypothesis might not have produced the same prediction. In the second case, as in a detective story, there are already enough facts, but several theories have failed to explain them. When a new hypothesis plausibly explains all of the facts, it may be quickly accepted before any further experiments are done. In the quantum-relativity revolution there are examples of both situations. Because of the two-stage development of both relativity (“special,” then “general”) and quantum theory (“old,” then “quantum mechanics”) in the period 1905-1930, we can make a double comparison of acceptance by prediction and by explanation. A curious anti- symmetry is revealed and discussed. _____________ *Distinguished University Professor (Emeritus) of the History of Science, University of Maryland. Home address: 108 Meadowlark Terrace, Glen Mills, PA 19342. Comments welcome. 1 “Science walks forward on two feet, namely theory and experiment. ... Sometimes it is only one foot which is put forward first, sometimes the other, but continuous progress is only made by the use of both – by theorizing and then testing, or by finding new relations in the process of experimenting and then bringing the theoretical foot up and pushing it on beyond, and so on in unending alterations.” Robert A. -

Quantum Mechanics I - Iii
QUANTUM MECHANICS I - III PHYS 516 - 518 Jan 1 - Dec. 31, 2015 Prof. R. Gilmore 12-918 X-2779 [email protected] office hours: 2:00 ! \1" Course Schedule: (Winter Quarter) MWF 11:00 - 11:50, Disque 919 Objective: To provide the foundations for modern physics. Course Requirements and Obligations Course grading will be based on assigned homework problem sets and a midterm and final exam. Texts Two texts and one supplement will be used for this course. The first text has been chosen from among many admirable texts because it provides a more comprehensive treatment of quantum physics discovered since 1970 than other texts. The second text will be used primarily during the second quarter of this course (PHYS517). It provides hands-on experience for solving binding and scattering problems in one dimension and potentials involving periodic poten- tials, again in one dimension. The third text (optional) is strongly recommended for those who feel their undergratuate experience in this beautiful subject may be deficient in some way. It is out of print but a limited number of copies are often available through Amazon in the event our book store has sold out of their reprinted copies. 1 David H. McIntyre Quantum Mechanics NY: Pearson, 2012 ISBN-10: 0-321-76579-6 R. Gilmore Elementary Quantum Mechanics in One Dimension Baltimore, Johns Hopkins University Press, 2004 ISBN 0-8018-8015-7 R. H. Dicke and J. P. Wittke Introduction to Quantum Mechanics Reading, MA: Addison-Wesley, 1960 ISBN 0-? If it becomes a hardship to acquire this fine text, you can get about the same information from another, later, fine text: David J. -
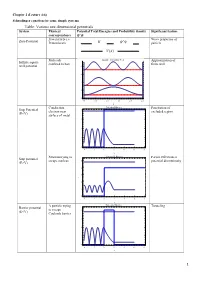
Table: Various One Dimensional Potentials System Physical Potential Total Energies and Probability Density Significant Feature Correspondence Free Particle I.E
Chapter 2 (Lecture 4-6) Schrodinger equation for some simple systems Table: Various one dimensional potentials System Physical Potential Total Energies and Probability density Significant feature correspondence Free particle i.e. Wave properties of Zero Potential Proton beam particle Molecule Infinite Potential Well Approximation of Infinite square confined to box finite well well potential 8 6 4 2 0 0.0 0.5 1.0 1.5 2.0 2.5 x Conduction Potential Barrier Penetration of Step Potential 4 electron near excluded region (E<V) surface of metal 3 2 1 0 4 2 0 2 4 6 8 x Neutron trying to Potential Barrier Partial reflection at Step potential escape nucleus 5 potential discontinuity (E>V) 4 3 2 1 0 4 2 0 2 4 6 8 x Α particle trying Potential Barrier Tunneling Barrier potential to escape 4 (E<V) Coulomb barrier 3 2 1 0 4 2 0 2 4 6 8 x 1 Electron Potential Barrier No reflection at Barrier potential 5 scattering from certain energies (E>V) negatively 4 ionized atom 3 2 1 0 4 2 0 2 4 6 8 x Neutron bound in Finite Potential Well Energy quantization Finite square well the nucleus potential 4 3 2 1 0 2 0 2 4 6 x Aromatic Degenerate quantum Particle in a ring compounds states contains atomic rings. Model the Quantization of energy Particle in a nucleus with a and degeneracy of spherical well potential which is or states zero inside the V=0 nuclear radius and infinite outside that radius. -

Chapter 2 Quantum Theory
Chapter 2 - Quantum Theory At the end of this chapter – the class will: Have basic concepts of quantum physical phenomena and a rudimentary working knowledge of quantum physics Have some familiarity with quantum mechanics and its application to atomic theory Quantization of energy; energy levels Quantum states, quantum number Implication on band theory Chapter 2 Outline Basic concept of quantization Origin of quantum theory and key quantum phenomena Quantum mechanics Example and application to atomic theory Concept introduction The quantum car Imagine you drive a car. You turn on engine and it immediately moves at 10 m/hr. You step on the gas pedal and it does nothing. You step on it harder and suddenly, the car moves at 40 m/hr. You step on the brake. It does nothing until you flatten the brake with all your might, and it suddenly drops back to 10 m/hr. What’s going on? Continuous vs. Quantization Consider a billiard ball. It requires accuracy and precision. You have a cue stick. Assume for simplicity that there is no friction loss. How fast can you make the ball move using the cue stick? How much kinetic energy can you give to the ball? The Newtonian mechanics answer is: • any value, as much as energy as you can deliver. The ball can be made moving with 1.000 joule, or 3.1415926535 or 0.551 … joule. Supposed it is moving with 1-joule energy, you can give it an extra 0.24563166 joule by hitting it with the cue stick by that amount of energy. -

Course of Study
Introduction to Quantum Chemistry and Spectroscopy CY41009; Autumn Semester 2016-2017 Course of Study 1. Birth of Quantum Mechanics Failures of Classical Mechanics in Black Body Radition, Photoelectric Effects, Heat capacity of Solids, and Atomic Spectra; Quantum Mechanics - the Saviour; Birth of Quantum Chemistry. 2. Principles and Postulates of Quantum Mechanics State Functions; Operators; Eigenfunctions; Expectation Values; Time Evolution of Expectation Values; Ehrenfest Theorem; Hermitian Property; Schmidt Orthogonalization; Dirac Notation; Dirac Delta Function; Commutation of Operators; Heisenberg's Uncertainty Principle; Parity Operator. 3. Exactly Solvable Models • Translational Motions Particle in a 1D box; Bohr's Correspondence Principle; Free Particle; Particle in a 3D box; Degeneracies; Particle in a Rectangular Well; Tunneling Through Barrier; Scanning Tunneling Microscopy. • Vibrational Motions Harmonic Oscillators; Creation-Annihilation Operators; Hermite Polynomials; Vibrational Spectroscopy • Angular Motions Angular Momentum Operators; Ladder Operators; Spherical Harmonics. • Rotational Motions Particle in a ring; Particle on a sphere; Rigid Rotor; • Hydrogen Atom Solution of H-atom; Bound-state H-atom Wave Functions; Radial Distribution Functions; H-like Orbitals; Zeeman Effect; H-atom with Electron Spin; Spin-Orbit Interaction; Atomic Spectra with Spin-Orbit Interac- tion in Magnetic Field. 4. Approximate Methods Variational Theorem; He atom with Variational Method; Perturbation Theory; 1st and 2nd Order Perturbation Correction