Chapter 2 Quantum Theory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unit 1 Old Quantum Theory
UNIT 1 OLD QUANTUM THEORY Structure Introduction Objectives li;,:overy of Sub-atomic Particles Earlier Atom Models Light as clectromagnetic Wave Failures of Classical Physics Black Body Radiation '1 Heat Capacity Variation Photoelectric Effect Atomic Spectra Planck's Quantum Theory, Black Body ~diation. and Heat Capacity Variation Einstein's Theory of Photoelectric Effect Bohr Atom Model Calculation of Radius of Orbits Energy of an Electron in an Orbit Atomic Spectra and Bohr's Theory Critical Analysis of Bohr's Theory Refinements in the Atomic Spectra The61-y Summary Terminal Questions Answers 1.1 INTRODUCTION The ideas of classical mechanics developed by Galileo, Kepler and Newton, when applied to atomic and molecular systems were found to be inadequate. Need was felt for a theory to describe, correlate and predict the behaviour of the sub-atomic particles. The quantum theory, proposed by Max Planck and applied by Einstein and Bohr to explain different aspects of behaviour of matter, is an important milestone in the formulation of the modern concept of atom. In this unit, we will study how black body radiation, heat capacity variation, photoelectric effect and atomic spectra of hydrogen can be explained on the basis of theories proposed by Max Planck, Einstein and Bohr. They based their theories on the postulate that all interactions between matter and radiation occur in terms of definite packets of energy, known as quanta. Their ideas, when extended further, led to the evolution of wave mechanics, which shows the dual nature of matter -
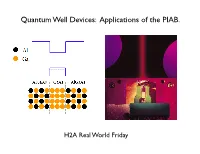
Quantum Well Devices: Applications of the PIAB
Quantum Well Devices: Applications of the PIAB. H2A Real World Friday What is a semiconductor? no e- Conduction Band Empty e levels a few e- Empty e levels lots of e- Empty e levels Filled e levels Filled e levels Filled e levels Insulator Semiconductor Metal Electrons in the conduction band of semiconductors like Si or GaAs can move about freely. Conduction band a few e- Eg = hν Energy Bandgap in Valence band Filled e levels Semiconductors We can get electrons into the conduction band by either thermal excitation or light excitation (photons). Solar cells use semiconductors to convert photons to electrons. A "quantum well" structure made from AlGaAs-GaAs-AlGaAs creates a potential well for conduction electrons. 10-20 nm! A conduction electron that get trapped in a quantum well acts like a PIAB. A conduction electron that get trapped in a quantum well acts like a PIAB. Quantum Wells are used to make Laser Diodes Quantum Well Laser Diodes Quantum Wells are used to make Laser Diodes Quantum Well Laser Diodes Multiple Quantum Wells work even better. Multiple Quantum Well Laser Diodes Multiple Quantum Wells work even better. Multiple Quantum Well LEDs Multiple Quantum Wells work even better. Multiple Quantum Well Laser Diodes Multiple Quantum Wells also are used to make high efficiency Solar Cells. Quantum Well Solar Cells Multiple Quantum Wells also are used to make high efficiency Solar Cells. The most common approach to high efficiency photovoltaic power conversion is to partition the solar spectrum into separate bands and each absorbed by a cell specially tailored for that spectral band. -
![Arxiv:1206.1084V3 [Quant-Ph] 3 May 2019](https://docslib.b-cdn.net/cover/2699/arxiv-1206-1084v3-quant-ph-3-may-2019-82699.webp)
Arxiv:1206.1084V3 [Quant-Ph] 3 May 2019
Overview of Bohmian Mechanics Xavier Oriolsa and Jordi Mompartb∗ aDepartament d'Enginyeria Electr`onica, Universitat Aut`onomade Barcelona, 08193, Bellaterra, SPAIN bDepartament de F´ısica, Universitat Aut`onomade Barcelona, 08193 Bellaterra, SPAIN This chapter provides a fully comprehensive overview of the Bohmian formulation of quantum phenomena. It starts with a historical review of the difficulties found by Louis de Broglie, David Bohm and John Bell to convince the scientific community about the validity and utility of Bohmian mechanics. Then, a formal explanation of Bohmian mechanics for non-relativistic single-particle quantum systems is presented. The generalization to many-particle systems, where correlations play an important role, is also explained. After that, the measurement process in Bohmian mechanics is discussed. It is emphasized that Bohmian mechanics exactly reproduces the mean value and temporal and spatial correlations obtained from the standard, i.e., `orthodox', formulation. The ontological characteristics of the Bohmian theory provide a description of measurements in a natural way, without the need of introducing stochastic operators for the wavefunction collapse. Several solved problems are presented at the end of the chapter giving additional mathematical support to some particular issues. A detailed description of computational algorithms to obtain Bohmian trajectories from the numerical solution of the Schr¨odingeror the Hamilton{Jacobi equations are presented in an appendix. The motivation of this chapter is twofold. -

Quantum Theory of the Hydrogen Atom
Quantum Theory of the Hydrogen Atom Chemistry 35 Fall 2000 Balmer and the Hydrogen Spectrum n 1885: Johann Balmer, a Swiss schoolteacher, empirically deduced a formula which predicted the wavelengths of emission for Hydrogen: l (in Å) = 3645.6 x n2 for n = 3, 4, 5, 6 n2 -4 •Predicts the wavelengths of the 4 visible emission lines from Hydrogen (which are called the Balmer Series) •Implies that there is some underlying order in the atom that results in this deceptively simple equation. 2 1 The Bohr Atom n 1913: Niels Bohr uses quantum theory to explain the origin of the line spectrum of hydrogen 1. The electron in a hydrogen atom can exist only in discrete orbits 2. The orbits are circular paths about the nucleus at varying radii 3. Each orbit corresponds to a particular energy 4. Orbit energies increase with increasing radii 5. The lowest energy orbit is called the ground state 6. After absorbing energy, the e- jumps to a higher energy orbit (an excited state) 7. When the e- drops down to a lower energy orbit, the energy lost can be given off as a quantum of light 8. The energy of the photon emitted is equal to the difference in energies of the two orbits involved 3 Mohr Bohr n Mathematically, Bohr equated the two forces acting on the orbiting electron: coulombic attraction = centrifugal accelleration 2 2 2 -(Z/4peo)(e /r ) = m(v /r) n Rearranging and making the wild assumption: mvr = n(h/2p) n e- angular momentum can only have certain quantified values in whole multiples of h/2p 4 2 Hydrogen Energy Levels n Based on this model, Bohr arrived at a simple equation to calculate the electron energy levels in hydrogen: 2 En = -RH(1/n ) for n = 1, 2, 3, 4, . -

The Concept of Quantum State : New Views on Old Phenomena Michel Paty
The concept of quantum state : new views on old phenomena Michel Paty To cite this version: Michel Paty. The concept of quantum state : new views on old phenomena. Ashtekar, Abhay, Cohen, Robert S., Howard, Don, Renn, Jürgen, Sarkar, Sahotra & Shimony, Abner. Revisiting the Founda- tions of Relativistic Physics : Festschrift in Honor of John Stachel, Boston Studies in the Philosophy and History of Science, Dordrecht: Kluwer Academic Publishers, p. 451-478, 2003. halshs-00189410 HAL Id: halshs-00189410 https://halshs.archives-ouvertes.fr/halshs-00189410 Submitted on 20 Nov 2007 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. « The concept of quantum state: new views on old phenomena », in Ashtekar, Abhay, Cohen, Robert S., Howard, Don, Renn, Jürgen, Sarkar, Sahotra & Shimony, Abner (eds.), Revisiting the Foundations of Relativistic Physics : Festschrift in Honor of John Stachel, Boston Studies in the Philosophy and History of Science, Dordrecht: Kluwer Academic Publishers, 451-478. , 2003 The concept of quantum state : new views on old phenomena par Michel PATY* ABSTRACT. Recent developments in the area of the knowledge of quantum systems have led to consider as physical facts statements that appeared formerly to be more related to interpretation, with free options. -

Dephasing in an Electronic Mach-Zehnder Interferometer,” Phys
Dephasing and Quantum Noise in an electronic Mach-Zehnder Interferometer Dissertation zur Erlangung des Doktorgrades der Naturwissenschaften (Dr. rer. nat.) der Fakultät für Physik der Universität Regensburg vorgelegt von Andreas Helzel aus Kelheim Dezember 2012 ii Promotionsgesuch eingereicht am: 22.11.2012 Die Arbeit wurde angeleitet von: Prof. Dr. Christoph Strunk Prüfungsausschuss: Prof. Dr. G. Bali (Vorsitzender) Prof. Dr. Ch. Strunk (1. Gutachter) Dr. F. Pierre (2. Gutachter) Prof. Dr. F. Gießibl (weiterer Prüfer) iii In Gedenken an Maria Höpfl. Sie war die erste Taxifahrerin von Kelheim und wurde 100 Jahre alt. Contents 1. Introduction1 2. Basics 5 2.1. The two dimensional electron gas....................5 2.2. The quantum Hall effect - Quantized Landau levels...........8 2.3. Transport in the quantum Hall regime.................. 10 2.3.1. Quantum Hall edge states and Landauer-Büttiker formalism.. 10 2.3.2. Compressible and incompressible strips............. 14 2.3.3. Luttinger liquid in the QH regime at filling factor 2....... 16 2.4. Non-equilibrium fluctuations of a QPC.................. 24 2.5. Aharonov-Bohm Interferometry..................... 28 2.6. The electronic Mach-Zehnder interferometer............... 30 3. Measurement techniques 37 3.1. Cryostat and devices........................... 37 3.2. Measurement approach.......................... 39 4. Sample fabrication and characterization 43 4.1. Fabrication................................ 43 4.1.1. Material.............................. 43 4.1.2. Lithography............................ 44 4.1.3. Gold air bridges.......................... 45 4.1.4. Sample Design.......................... 46 4.2. Characterization.............................. 47 4.2.1. Filling factor........................... 47 4.2.2. Quantum point contacts..................... 49 4.2.3. Gate setting............................ 50 5. Characteristics of a MZI 51 5.1. -

Vibrational Quantum Number
Fundamentals in Biophotonics Quantum nature of atoms, molecules – matter Aleksandra Radenovic [email protected] EPFL – Ecole Polytechnique Federale de Lausanne Bioengineering Institute IBI 26. 03. 2018. Quantum numbers •The four quantum numbers-are discrete sets of integers or half- integers. –n: Principal quantum number-The first describes the electron shell, or energy level, of an atom –ℓ : Orbital angular momentum quantum number-as the angular quantum number or orbital quantum number) describes the subshell, and gives the magnitude of the orbital angular momentum through the relation Ll2 ( 1) –mℓ:Magnetic (azimuthal) quantum number (refers, to the direction of the angular momentum vector. The magnetic quantum number m does not affect the electron's energy, but it does affect the probability cloud)- magnetic quantum number determines the energy shift of an atomic orbital due to an external magnetic field-Zeeman effect -s spin- intrinsic angular momentum Spin "up" and "down" allows two electrons for each set of spatial quantum numbers. The restrictions for the quantum numbers: – n = 1, 2, 3, 4, . – ℓ = 0, 1, 2, 3, . , n − 1 – mℓ = − ℓ, − ℓ + 1, . , 0, 1, . , ℓ − 1, ℓ – –Equivalently: n > 0 The energy levels are: ℓ < n |m | ≤ ℓ ℓ E E 0 n n2 Stern-Gerlach experiment If the particles were classical spinning objects, one would expect the distribution of their spin angular momentum vectors to be random and continuous. Each particle would be deflected by a different amount, producing some density distribution on the detector screen. Instead, the particles passing through the Stern–Gerlach apparatus are deflected either up or down by a specific amount. -

Lecture 3: Particle in a 1D Box
Lecture 3: Particle in a 1D Box First we will consider a free particle moving in 1D so V (x) = 0. The TDSE now reads ~2 d2ψ(x) = Eψ(x) −2m dx2 which is solved by the function ψ = Aeikx where √2mE k = ± ~ A general solution of this equation is ψ(x) = Aeikx + Be−ikx where A and B are arbitrary constants. It can also be written in terms of sines and cosines as ψ(x) = C sin(kx) + D cos(kx) The constants appearing in the solution are determined by the boundary conditions. For a free particle that can be anywhere, there is no boundary conditions, so k and thus E = ~2k2/2m can take any values. The solution of the form eikx corresponds to a wave travelling in the +x direction and similarly e−ikx corresponds to a wave travelling in the -x direction. These are eigenfunctions of the momentum operator. Since the particle is free, it is equally likely to be anywhere so ψ∗(x)ψ(x) is independent of x. Incidently, it cannot be normalized because the particle can be found anywhere with equal probability. 1 Now, let us confine the particle to a region between x = 0 and x = L. To do this, we choose our interaction potential V (x) as follows V (x) = 0 for 0 x L ≤ ≤ = otherwise ∞ It is always a good idea to plot the potential energy, when it is a function of a single variable, as shown in Fig.1. The TISE is now given by V(x) V=infinity V=0 V=infinity x 0 L ~2 d2ψ(x) + V (x)ψ(x) = Eψ(x) −2m dx2 First consider the region outside the box where V (x) = . -

The Quantum Mechanical Model of the Atom
The Quantum Mechanical Model of the Atom Quantum Numbers In order to describe the probable location of electrons, they are assigned four numbers called quantum numbers. The quantum numbers of an electron are kind of like the electron’s “address”. No two electrons can be described by the exact same four quantum numbers. This is called The Pauli Exclusion Principle. • Principle quantum number: The principle quantum number describes which orbit the electron is in and therefore how much energy the electron has. - it is symbolized by the letter n. - positive whole numbers are assigned (not including 0): n=1, n=2, n=3 , etc - the higher the number, the further the orbit from the nucleus - the higher the number, the more energy the electron has (this is sort of like Bohr’s energy levels) - the orbits (energy levels) are also called shells • Angular momentum (azimuthal) quantum number: The azimuthal quantum number describes the sublevels (subshells) that occur in each of the levels (shells) described above. - it is symbolized by the letter l - positive whole number values including 0 are assigned: l = 0, l = 1, l = 2, etc. - each number represents the shape of a subshell: l = 0, represents an s subshell l = 1, represents a p subshell l = 2, represents a d subshell l = 3, represents an f subshell - the higher the number, the more complex the shape of the subshell. The picture below shows the shape of the s and p subshells: (notice the electron clouds) • Magnetic quantum number: All of the subshells described above (except s) have more than one orientation. -

Walther Nernst, Albert Einstein, Otto Stern, Adriaan Fokker
Walther Nernst, Albert Einstein, Otto Stern, Adriaan Fokker, and the Rotational Specific Heat of Hydrogen Clayton Gearhart St. John’s University (Minnesota) Max-Planck-Institut für Wissenschaftsgeschichte Berlin July 2007 1 Rotational Specific Heat of Hydrogen: Widely investigated in the old quantum theory Nernst Lorentz Eucken Einstein Ehrenfest Bohr Planck Reiche Kemble Tolman Schrödinger Van Vleck Some of the more prominent physicists and physical chemists who worked on the specific heat of hydrogen through the mid-1920s. See “The Rotational Specific Heat of Molecular Hydrogen in the Old Quantum Theory” http://faculty.csbsju.edu/cgearhart/pubs/sel_pubs.htm (slide show) 2 Rigid Rotator (Rotating dumbbell) •The rigid rotator was among the earliest problems taken up in the old (pre-1925) quantum theory. • Applications: Molecular spectra, and rotational contri- bution to the specific heat of molecular hydrogen • The problem should have been simple Molecular • relatively uncomplicated theory spectra • only one adjustable parameter (moment of inertia) • Nevertheless, no satisfactory theoretical description of the specific heat of hydrogen emerged in the old quantum theory Our story begins with 3 Nernst’s Heat Theorem Walther Nernst 1864 – 1941 • physical chemist • studied with Boltzmann • 1889: lecturer, then professor at Göttingen • 1905: professor at Berlin Nernst formulated his heat theorem (Third Law) in 1906, shortly after appointment as professor in Berlin. 4 Nernst’s Heat Theorem and Quantum Theory • Initially, had nothing to do with quantum theory. • Understand the equilibrium point of chemical reactions. • Nernst’s theorem had implications for specific heats at low temperatures. • 1906–1910: Nernst and his students undertook extensive measurements of specific heats of solids at low (down to liquid hydrogen) temperatures. -

Entanglement of the Uncertainty Principle
Open Journal of Mathematics and Physics | Volume 2, Article 127, 2020 | ISSN: 2674-5747 https://doi.org/10.31219/osf.io/9jhwx | published: 26 Jul 2020 | https://ojmp.org EX [original insight] Diamond Open Access Entanglement of the Uncertainty Principle Open Quantum Collaboration∗† August 15, 2020 Abstract We propose that position and momentum in the uncertainty principle are quantum entangled states. keywords: entanglement, uncertainty principle, quantum gravity The most updated version of this paper is available at https://osf.io/9jhwx/download Introduction 1. [1] 2. Logic leads us to conclude that quantum gravity–the theory of quan- tum spacetime–is the description of entanglement of the quantum states of spacetime and of the extra dimensions (if they really ex- ist) [2–7]. ∗All authors with their affiliations appear at the end of this paper. †Corresponding author: [email protected] | Join the Open Quantum Collaboration 1 Quantum Gravity 3. quantum gravity quantum spacetime entanglement of spacetime 4. Quantum spaceti=me might be in a sup=erposition of the extra dimen- sions [8]. Notation 5. UP Uncertainty Principle 6. UP= the quantum state of the uncertainty principle ∣ ⟩ = Discussion on the notation 7. The universe is mathematical. 8. The uncertainty principle is part of our universe, thus, it is described by mathematics. 9. A physical theory must itself be described within its own notation. 10. Thereupon, we introduce UP . ∣ ⟩ Entanglement 11. UP α1 x1p1 α2 x2p2 α3 x3p3 ... 12. ∣xi ⟩u=ncer∣tainty⟩ +in po∣ sitio⟩n+ ∣ ⟩ + 13. pi = uncertainty in momentum 14. xi=pi xi pi xi pi ∣ ⟩ = ∣ ⟩ ∣ ⟩ = ∣ ⟩ ⊗ ∣ ⟩ 2 Final Remarks 15. -

Path Integral for the Hydrogen Atom
Path Integral for the Hydrogen Atom Solutions in two and three dimensions Vägintegral för Väteatomen Lösningar i två och tre dimensioner Anders Svensson Faculty of Health, Science and Technology Physics, Bachelor Degree Project 15 ECTS Credits Supervisor: Jürgen Fuchs Examiner: Marcus Berg June 2016 Abstract The path integral formulation of quantum mechanics generalizes the action principle of classical mechanics. The Feynman path integral is, roughly speaking, a sum over all possible paths that a particle can take between fixed endpoints, where each path contributes to the sum by a phase factor involving the action for the path. The resulting sum gives the probability amplitude of propagation between the two endpoints, a quantity called the propagator. Solutions of the Feynman path integral formula exist, however, only for a small number of simple systems, and modifications need to be made when dealing with more complicated systems involving singular potentials, including the Coulomb potential. We derive a generalized path integral formula, that can be used in these cases, for a quantity called the pseudo-propagator from which we obtain the fixed-energy amplitude, related to the propagator by a Fourier transform. The new path integral formula is then successfully solved for the Hydrogen atom in two and three dimensions, and we obtain integral representations for the fixed-energy amplitude. Sammanfattning V¨agintegral-formuleringen av kvantmekanik generaliserar minsta-verkanprincipen fr˚anklassisk meka- nik. Feynmans v¨agintegral kan ses som en summa ¨over alla m¨ojligav¨agaren partikel kan ta mellan tv˚a givna ¨andpunkterA och B, d¨arvarje v¨agbidrar till summan med en fasfaktor inneh˚allandeden klas- siska verkan f¨orv¨agen.Den resulterande summan ger propagatorn, sannolikhetsamplituden att partikeln g˚arfr˚anA till B.