Aniridia [Includes: Isolated Aniridia, Wilms Tumor- Aniridia-Genital Anomalies-Retardation (WAGR) Syndrome]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insertion of Aqueous Shunt in Pedicatric Glaucoma
1/29/2018 Challenges of Insertion of Aqueous shunt in paediatric glaucoma Ahmed Elkarmouty MD, FRCS Moorfields Eye Hospital London, UK Classification • Primary Childhood Glaucoma • A- Primary Congenital Glaucoma (PCG) 1: 10,000–18,000 • B- Juvenile Open Angle Glaucoma (JOAG) (5-35 ys,)1 : 50,000. • Secondary Childhood Glaucoma • A- Glaucoma associated with non-acquired ocular anomalies • B- Glaucoma associated with non- acquired systemic disease or syndrome • C- Glaucoma associated with acquired condition • D- Glaucoma following Cataract surgery 1 1/29/2018 Glaucoma associated with non- acquired ocular anomalies • Conditions with predominantly ocular anomalies present at birth which may or may not be associated with systemic signs • Axenfeld Reiger anomaly • Peters anomaly • Ectropion Uvae • Congenital iris hypolplasia • Aniridia • Oculodermal melanocytosis • Posterior polymorphous dystrophy • Microphthalmos • Microcornea • Ectopia Lentis ( et pupillae) • Persistent foetus vasculopathy Glaucoma associated with non- acquired systemic disease or syndrome predominantly associated with known syndrome, systemic anomalies present at birth which may be associated with ocular signs • Down Syndrome • Connective tissue disorder: Marfan syndrome, Weill- Marchesiani syndrome, Stickler syndrome • Metabolic disorder : Homocystenuria, lowe syndrome, Mucoploysacchroidoses • Phacomatoses: Neurofibromatoses, Sturge Weber, Klipple-Trenaunay- weber syndrome, Rubenstein Taybi • Congenital Rubella 2 1/29/2018 Glaucoma associated with acquired condition Conditions -

Expanding the Phenotypic Spectrum of PAX6 Mutations: from Congenital Cataracts to Nystagmus
G C A T T A C G G C A T genes Article Expanding the Phenotypic Spectrum of PAX6 Mutations: From Congenital Cataracts to Nystagmus Maria Nieves-Moreno 1,* , Susana Noval 1 , Jesus Peralta 1, María Palomares-Bralo 2 , Angela del Pozo 3 , Sixto Garcia-Miñaur 4, Fernando Santos-Simarro 4 and Elena Vallespin 5 1 Department of Ophthalmology, Hospital Universitario La Paz, 28046 Madrid, Spain; [email protected] (S.N.); [email protected] (J.P.) 2 Department of Molecular Developmental Disorders, Medical and Molecular Genetics Institue (INGEMM) IdiPaz, CIBERER, Hospital Universitario La Paz, 28046 Madrid, Spain; [email protected] 3 Department of Bioinformatics, Medical and Molecular Genetics Institue (INGEMM) IdiPaz, CIBERER, Hospital Universitario La Paz, 28046 Madrid, Spain; [email protected] 4 Department of Clinical Genetics, Medical and Molecular Genetics Institue (INGEMM) IdiPaz, CIBERER, Hospital Universitario La Paz, 28046 Madrid, Spain; [email protected] (S.G.-M.); [email protected] (F.S.-S.) 5 Department of Molecular Ophthalmology, Medical and Molecular Genetics Institue (INGEMM) IdiPaz, CIBERER, Hospital Universitario La Paz, 28046 Madrid, Spain; [email protected] * Correspondence: [email protected] Abstract: Background: Congenital aniridia is a complex ocular disorder, usually associated with severe visual impairment, generally caused by mutations on the PAX6 gene. The clinical phenotype of PAX6 mutations is highly variable, making the genotype–phenotype correlations difficult to establish. Methods: we describe the phenotype of eight patients from seven unrelated families Citation: Nieves-Moreno, M.; Noval, with confirmed mutations in PAX6, and very different clinical manifestations. -

WAGR Syndrome: Clinical Features and Guidelines for Management
WAGR syndrome: clinical features and guidelines for management Kelly Trout, BSN, RN International WAGR Syndrome Association 2020 Introduction WAGR syndrome is a rare multiple congenital-anomaly syndrome caused by interstitial deletion of the distal portion of chromosome 11p13. Deletion size varies between individuals from 1 million to 26.5 million base pairs, with an average of 11 million base pairs. Variability in size of the genetic deletion is thought to account for the variable phenotype. WAGR is an acronym for the most prominent features: W is for Wilms tumor, A for aniridia, G for genitourinary anomalies, and R for range of developmental delays. Wilms tumor and genital anomalies are caused by deletion of the WT1 tumor-suppressor gene, and aniridia is caused by deletion of the PAX6 ocular development gene. Developmental delays are presumed to be the result of deletion of as yet unidentified genes in the region. Most cases are identified by chromosome studies of children with isolated aniridia and are due to de novo deletions, although a few familial translocations have been reported. Individuals with WAGR syndrome have a high risk for development of Wilms tumor and late-onset renal failure, as well as a variety of additional associated conditions. Diagnosis ● Most cases of WAGR syndrome are identified in infants with isolated aniridia, 30% of whom will be positive for the characteristic deletion (11p13) ● In rare cases, aniridia may not be present. Children with Wilms tumor and genital anomalies may also warrant genetic testing ● -
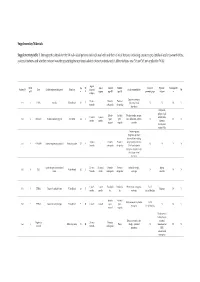
Solved/Unsolved
Supplementary Materials: Supplementary table 1. Demographic details for the 54 individual patients (solved/unsolved) and their clinical features including cataract type, details of ocular co-morbidities, systemic features and whether cataract was the presenting feature (non-isolated cataract patients only). Abbreviations: yes (Y), no (N), not applicable (N/A). Age at Famil Ag M/ Age at Cataract Cataract Cataract Systemic Consanguinit Patient ID Gene Confirmed genetic diagnosis Ethnicity diagnosi Ocular co-morbidities FH y ID e F surgery type RE type LE presenting sign features y s (days) Aniridia, nystagmus, 23 years Posterior Posterior 1-1 1 PAX6 Aniridia White British 25 F - glaucoma, foveal N N N Y 4 months subcapsular subcapsular hypoplasia Cleft palate, epilepsy, high Aphakia Aphakia Macular atrophy, myopia, 7 years 9 7 years 8 arched palate, 2-1 2 COL11A1 Stickler syndrome, type II Not Stated 34 F (post- (post- lens subluxation, vitreous N N N months months flattened surgical) surgical) anomaly maxilla, short stature (5'2ft) Anterior segment dysgenesis, pupillary abnormalities including 12 years Posterior Posterior ectopic pupils, ectropion 3-1 3 CPAMD8 Anterior segment dysgenesis 8 Other, Any other 27 F - N N Y N 5 months subcapsular subcapsular UVAE and irodensis, nystagmus, dysplastic optic discs, large corneal diameters Gyrate atrophy of choroid and 23 years 29 years 1 Posterior Posterior Retinal dystrophy, Bipolar 4-1 4 OAT White British 42 F N N N retina 7 months month subcapsular subcapsular exotropia disorder 1 year 6 1 year -

MECHANISMS in ENDOCRINOLOGY: Novel Genetic Causes of Short Stature
J M Wit and others Genetics of short stature 174:4 R145–R173 Review MECHANISMS IN ENDOCRINOLOGY Novel genetic causes of short stature 1 1 2 2 Jan M Wit , Wilma Oostdijk , Monique Losekoot , Hermine A van Duyvenvoorde , Correspondence Claudia A L Ruivenkamp2 and Sarina G Kant2 should be addressed to J M Wit Departments of 1Paediatrics and 2Clinical Genetics, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, Email The Netherlands [email protected] Abstract The fast technological development, particularly single nucleotide polymorphism array, array-comparative genomic hybridization, and whole exome sequencing, has led to the discovery of many novel genetic causes of growth failure. In this review we discuss a selection of these, according to a diagnostic classification centred on the epiphyseal growth plate. We successively discuss disorders in hormone signalling, paracrine factors, matrix molecules, intracellular pathways, and fundamental cellular processes, followed by chromosomal aberrations including copy number variants (CNVs) and imprinting disorders associated with short stature. Many novel causes of GH deficiency (GHD) as part of combined pituitary hormone deficiency have been uncovered. The most frequent genetic causes of isolated GHD are GH1 and GHRHR defects, but several novel causes have recently been found, such as GHSR, RNPC3, and IFT172 mutations. Besides well-defined causes of GH insensitivity (GHR, STAT5B, IGFALS, IGF1 defects), disorders of NFkB signalling, STAT3 and IGF2 have recently been discovered. Heterozygous IGF1R defects are a relatively frequent cause of prenatal and postnatal growth retardation. TRHA mutations cause a syndromic form of short stature with elevated T3/T4 ratio. Disorders of signalling of various paracrine factors (FGFs, BMPs, WNTs, PTHrP/IHH, and CNP/NPR2) or genetic defects affecting cartilage extracellular matrix usually cause disproportionate short stature. -

Journal of Ophthalmology & Clinical Research
ISSN: 2573-9573 Case Report Journal of Ophthalmology & Clinical Research Bilateral Congenital Ectropion Uveae, Anterior Segment Dysgenesis and Aniridia with Microspherophakic Congenital Cataracts and RubeosisIridis Rao Muhammad Arif Khan* and Ashal Kaiser Pal *Corresponding author Rao Muhammad Arif Khan, MCPS, FCPS, FPO, FACS, Pediatric Ophthalmologist, King Edward Medical University, Al-Awali Street, Taif Road, Makkah, Saudi Arabia, Pediatric Ophthalmologist, King Edward Medical University, Tel: 00966560479694; E-mail: [email protected] Makkah, Saudi Arabia Submitted: 02 Apr 2018; Accepted: 12 Apr 2018; Published: 19 Apr 2018 Abstract In recent times, multiple eye diseases have been seen associated with an increase in the rate of Demodex infestation as a possible cause, but in the particular case of dry eye syndrome in patients treated with platelet-rich plasma, this increase in mite may be relevant to guide a more adequate treatment focusing on the elimination of the mite in conjunction with the recovery of the ocular ecology. The demodex mite is a commensal parasite that lives in hair follicles, sebaceous glands and meibomian, which in a high rate of infestation can generate alterations in the ocular area. Performing an adequate diagnosis for the detection of the mite and treatment for its eradication can be effective for the recovery of the normal physiology of the tear film that constitutes a cause of dry eye. Introduction Congenital ectropion uvea is a rare ocular manifestation of neural crest syndrome [1]. It is a non-progressive anomaly characterized by presence of iris pigment epithelium on anterior surface of iris from the pigment ruff [2]. Congenital glaucoma is its common association [3-8]. -
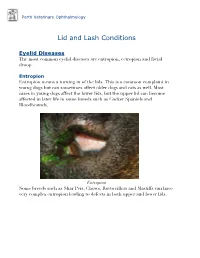
Lid and Lash Conditions
Perth Veterinary Ophthalmology Lid and Lash Conditions Eyelid Diseases The most common eyelid diseases are entropion, ectropion and facial droop. Entropion Entropion means a turning in of the lids. This is a common complaint in young dogs but can sometimes affect older dogs and cats as well. Most cases in young dogs affect the lower lids, but the upper lid can become affected in later life in some breeds such as Cocker Spaniels and Bloodhounds. Entropion Some breeds such as Shar Peis, Chows, Rottweillers and Mastiffs can have very complex entropion leading to defects in both upper and lower lids. A Shar Pei with severe upper and lower lid entropion Entropion is painful and can be potentially blinding. The rolling in of the lid leads to hair coming into contact with the cornea, leading to pain, ulceration and scarring (which can affect vision). In severe cases this can even lead to perforation of the eye. There are many causes of entropion. It can be primary or secondary to other problems affecting the lids (such as ectopic cilia, distichiasis etc. - see below). Some possible causes include the lid being too long, the lid being too tight, instability of the lateral canthus (outer cornea of the eyelids), misdirection of the lateral canthal tendon, brachycephalic anatomy (big eyes and short nose - e.g. Pekingese, Pugs, Shih Tsus, Persian cats etc.), diamond eye defects, loose or too much skin, facial droop etc. Often these cases are referred to a veterinary ophthalmologist for proper assessment and treatment to provide the best outcome. Entropion requires surgical correction. -

Congenital Ocular Anomalies in Newborns: a Practical Atlas
www.jpnim.com Open Access eISSN: 2281-0692 Journal of Pediatric and Neonatal Individualized Medicine 2020;9(2):e090207 doi: 10.7363/090207 Received: 2019 Jul 19; revised: 2019 Jul 23; accepted: 2019 Jul 24; published online: 2020 Sept 04 Mini Atlas Congenital ocular anomalies in newborns: a practical atlas Federico Mecarini1, Vassilios Fanos1,2, Giangiorgio Crisponi1 1Neonatal Intensive Care Unit, Azienda Ospedaliero-Universitaria Cagliari, University of Cagliari, Cagliari, Italy 2Department of Surgery, University of Cagliari, Cagliari, Italy Abstract All newborns should be examined for ocular structural abnormalities, an essential part of the newborn assessment. Early detection of congenital ocular disorders is important to begin appropriate medical or surgical therapy and to prevent visual problems and blindness, which could deeply affect a child’s life. The present review aims to describe the main congenital ocular anomalies in newborns and provide images in order to help the physician in current clinical practice. Keywords Congenital ocular anomalies, newborn, anophthalmia, microphthalmia, aniridia, iris coloboma, glaucoma, blepharoptosis, epibulbar dermoids, eyelid haemangioma, hypertelorism, hypotelorism, ankyloblepharon filiforme adnatum, dacryocystitis, dacryostenosis, blepharophimosis, chemosis, blue sclera, corneal opacity. Corresponding author Federico Mecarini, MD, Neonatal Intensive Care Unit, Azienda Ospedaliero-Universitaria Cagliari, University of Cagliari, Cagliari, Italy; tel.: (+39) 3298343193; e-mail: [email protected]. -

Eleventh Edition
SUPPLEMENT TO April 15, 2009 A JOBSON PUBLICATION www.revoptom.com Eleventh Edition Joseph W. Sowka, O.D., FAAO, Dipl. Andrew S. Gurwood, O.D., FAAO, Dipl. Alan G. Kabat, O.D., FAAO Supported by an unrestricted grant from Alcon, Inc. 001_ro0409_handbook 4/2/09 9:42 AM Page 4 TABLE OF CONTENTS Eyelids & Adnexa Conjunctiva & Sclera Cornea Uvea & Glaucoma Viitreous & Retiina Neuro-Ophthalmic Disease Oculosystemic Disease EYELIDS & ADNEXA VITREOUS & RETINA Blow-Out Fracture................................................ 6 Asteroid Hyalosis ................................................33 Acquired Ptosis ................................................... 7 Retinal Arterial Macroaneurysm............................34 Acquired Entropion ............................................. 9 Retinal Emboli.....................................................36 Verruca & Papilloma............................................11 Hypertensive Retinopathy.....................................37 Idiopathic Juxtafoveal Retinal Telangiectasia...........39 CONJUNCTIVA & SCLERA Ocular Ischemic Syndrome...................................40 Scleral Melt ........................................................13 Retinal Artery Occlusion ......................................42 Giant Papillary Conjunctivitis................................14 Conjunctival Lymphoma .......................................15 NEURO-OPHTHALMIC DISEASE Blue Sclera .........................................................17 Dorsal Midbrain Syndrome ..................................45 -

Aniridia in Children
Aniridia in Children This material will help you understand aniridia and how it is treated. What is aniridia? Aniridia is an eye disorder where the iris is completely or partially absent. The iris is the colored part of the eye that surrounds the black pupil. People with aniridia have large pupils. The condition affects both eyes. How does aniridia affect my child’s eyesight? Aniridia alone does not affect your child’s visual acuity. Some children with aniridia have very good vision (20/30 or better) whereas others have very poor vision (20/200 or worse). However, because aniridia causes the pupils to be large, your child may have sensitivity to light and glare. Aniridia is also associated with other eye conditions including: • Cataracts (clouding of the eye lens) • Glaucoma (increased eye pressure) • Nystagmus (wobbly eyes) • Scarring of the cornea (the front of the eye) What causes aniridia? Aniridia is caused by a genetic mutation. The mutation affects the PAX6 gene, which is involved in the early development of the eyes, brain, spinal cord, and pancreas. There are different ways that the mutation can be inherited. In some cases, aniridia is part of a syndrome. A syndrome is a group of medical conditions that occur together. The following syndromes involve aniridia: • Miller syndrome (aniridia and Wilm’s (kidney) tumor) • WAGR syndrome (Wilm’s (kidney) tumor, aniridia, genital abnormalities, and mental retardation) Kellogg Eye Center Aniridia 1 • Gillepsie’s syndrome (aniridia, mental retardation, and balance problems) How is aniridia treated? Glasses or contacts can help your child see more clearly. There are special types of glasses and contacts available that can also help reduce sensitivity to light. -

Frasier Syndrome: a Cause of Focal Segmental Glomerulosclerosis in a 46,XX Female
BRIEF COMMUNICATIONS J Am Soc Nephrol 10: 2215–2218, 1999 Frasier Syndrome: A Cause of Focal Segmental Glomerulosclerosis in a 46,XX Female LAURIE DEMMER,* WILLIAM PRIMACK,*† VALERIE LOIK,‡ ROSALIND BROWN,* NICOLE THERVILLE,§ and KEN MCELREAVEY§ *Department of Pediatrics, University of Massachusetts Memorial Health Care, Worcester, Massachusetts; †Fallon Clinic, Worcester, Massachusetts; ‡Department of Obstetrics & Gynecology, Baystate Medical Center, Springfield, Massachusetts; and §Department of Human Immunogenetics, Institut Pasteur, Paris, France. Abstract. The description of Frasier syndrome until now has diagnosis of Frasier syndrome with 46,XY gonadal dysgenesis, been restricted to XY females with gonadal dysgenesis, pro- whereas her sister has progressive glomerulopathy but a 46,XX gressive glomerulopathy, and a significant risk of gonadoblas- karyotype and normal female development. This indicates that toma. Mutations in the donor splice site in intron 9 of the the proper WT1 isoform ratio is critical for renal and testicular Wilms’ tumor (WT1) gene have been shown to cause Frasier development, but apparently does not affect either ovarian syndrome and are distinct from WT1 exon mutations associ- development or function. It is proposed that the clinical defi- ated with Denys-Drash syndrome. The WT1 gene, which is nition of Frasier syndrome should be broadened to include essential for normal kidney and gonadal development, encodes 46,XX females with normal genital development and focal a zinc finger transcription factor. The intron 9 alternative splice segmental glomerulosclerosis associated with a WT1 intron 9 donor site mutation seen in Frasier syndrome leads to loss of donor splice site mutation. Nephrologists need to consider the three amino acids (1KTS isoform), thus disrupting the normal possibility of this heritable syndrome in evaluation of females ratio of the 1KTS/2KTS isoforms critical for proper gonadal with focal segmental glomerulosclerosis and to consider their and renal development. -

WAGR Syndrome
International J. of Healthcare and Biomedical Research, Volume: 2, Issue: 4, July 2014, Pages 131-134 Case Report: WAGR Syndrome 1Dr.Prathamesh Patil , 2Dr. C Ashok kumar, 3Dr. Shashank Panwar , 4Dr. R Somdeepak Reddy , 5Dr. Pratik Gogri 1Junior Resident, Pediatrics, Pravara Institute of Medical sciences, Loni, Maharashtra, India 2 Associate Professor of Pediatrics, Pravara Institute of Medical sciences, Loni, Maharashtra, India 3 Junior Resident, Pediatrics, Pravara Institute of Medical sciences, Loni, Maharashtra, India 4 Junior Resident, Pediatrics, Pravara Institute of Medical sciences, Loni, Maharashtra, India 5 Junior Resident, Opthalmology, Pravara Institute of Medical sciences, Loni, Maharashtra, India Corresponding author: Dr. Prathamesh Patil; Email: [email protected] Abstract: WAGR Syndrome is a multiple congenital anomaly syndrome characterized by interstitial deletion of distal portion of chromosome 11p13. It is a gene deletion syndrome. WAGR is an acronym for W- Wilms tumor, A- aniridia, G- genital anomalies and R- mental retardation. Wilms tumor and male genital anomalies are caused by deletion of the WT1 tumor suppressor gene, and aniridia is caused by deletion of PAX6 ocular developmental gene. Mental retardation is thought to be a due of deletion of multiple as yet unidentified genes in the region. The patient needs a multidisciplinary management regimen encompassing the services of an ophthalmologist, radiologist, a pediatrician and pediatric surgeon. Herewith we reported a patient of developmental delay with abdominal distension, child also had aniridia. The child was managed by multidisciplinary approach. Key words: WAGR Syndrome Background: she was found to have aniridia, Wilms tumor and WAGR Syndrome is a multiple congenital anomaly mental retardation. The findings that aniridia is syndrome characterized by interstitial deletion of associated with Wilms tumor made that the findings distal portion of chromosome 11p13.