Complementary Roles of Neurotrophin 3 and a N-Methyl-D-Aspartate Antagonist in the Protection of Noise and Aminoglycoside- Induced Ototoxicity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fibroblast Growth Factor (Fgf) Implication in the Neonatal Development of the Cochlear Innervation G
FIBROBLAST GROWTH FACTOR (FGF) IMPLICATION IN THE NEONATAL DEVELOPMENT OF THE COCHLEAR INNERVATION G. Després, I. Jalenques, R. Romand To cite this version: G. Després, I. Jalenques, R. Romand. FIBROBLAST GROWTH FACTOR (FGF) IMPLICATION IN THE NEONATAL DEVELOPMENT OF THE COCHLEAR INNERVATION. Journal de Physique IV Proceedings, EDP Sciences, 1992, 02 (C1), pp.C1-173-C1-176. 10.1051/jp4:1992134. jpa-00251205 HAL Id: jpa-00251205 https://hal.archives-ouvertes.fr/jpa-00251205 Submitted on 1 Jan 1992 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. JOURNAL DE PHYSIQUE IV Colloque C1, suppICment au Journal de Physique 111, Volume 2, avril 1992 FIBROBLAST GROWMI FACTOR (FGF) IMPLICATION IN TIlE NEONATAL DEVELOPMENT OF THE COCIiLEAR INNERVATION G. DESPR~S,I. JALENQUES and R. ROMAND Laboratoire de Neurobiolc@e et Physwlogie du Dkeloppement, Universite'BIaise Pascal, F-63177Aubi2re ceder, France The presence of fibroblast growth factor-like protein has been investigated on cryostat sections from Sprague Dawley rat cochleae and auditory brainstem nuclei of various neonatal stages by indirect immunofluorescence and immunoperoxydase techniques with an antibody directed against the 1-24 amino-acid sequence of brain derived basic FGF. -

Hearing Loss Epidemic the Hair Cell
Hearing loss epidemic One in ten (30 million) Americans has hearing loss FUTURE THERAPIES FOR INNER - Causes include heredity, aging, noise exposure, disease EAR REGENERATION - Number is expected to double by 2030 Hearing loss is the #1 birth defect in America Albert Edge - 1 in 1000 newborns is born profoundly deaf Harvard Medical School - 2-3/1000 will have partial/progressive hearing loss Massachusetts Eye and Ear Infirmary Hearing loss prevalence increases with age - 1 in 3 over 65 years has significant hearing loss - Among seniors, hearing loss is the 3rd most prevalent condition 2 The inner ear The hair cell Auditory Hair Bundle Nerve Middle Ear Sensory hairs vibrate, "tip-links"open ion channels into hair cell Ions flow into hair cell, Inner Ear changing its electrical potential Hair External Ear Cells 3 4 1 The nerve fiber Sensorineural hearing loss: Hair cells and nerve fibers Cochlear Implant can directly stimulate Electric potential causes chemical neurotransmitter release from synapse Sensory Cell Loss NeurotransmitterNeurotransmitter diffuses to nerve fiber and excites electrical activity in the form of action potentials Hair Cell Nerve Fiber Loss 5 6 Regeneration of hair cells in chick inner ear Can stem cell-derived inner ear progenitors replace lost hair cells in vivo (and restore hearing)? Normal Hair Cells Damaged Hair Cells Regenerated Hair Cell Bundles Li et al., TMM (2004) 2 Approaches to regenerating inner ear cells Gene therapy I. Generation of inner ear cells by gene therapy • New hair cells: transfer Atoh1 gene II. -

5.1. Structure of the Spiral Ganglion
CHAPTER 5. INNERVATION OF THE ORGAN OF CORTI The investigation of nerve components of the acoustic system’s periph- eral part is so difficult methodologically that a whole series of questions which were solved long ago during the investigation of the other sensory system have not yet been clarified. The basic difficulty for the morphologists lies in the fact that the organ of Corti, together with its nerve elements, is located within the osseous tissue. In addition, it is in the shape of a spirally involut- ed geometrical figure. These structural peculiarities create considerable dif- ficulties during the determination of the connections between the types of peripheral and central neuron’s processes and bodies of the spiral ganglion of the cochlea. Therefore, most of the work on the cochlea’s innervation and the computation of the different element’s quantity demands an application of special methods, including graphical reconstruction of the serial sections. The Golgi method was and still remains the basic histological method of the organ of Corti’s innervation study, which has been supplemented by the cochlea’s electron-microscope investigations in the normal conditions and during the experimentally induced degenerations. 5.1. Structure of the spiral ganglion The neurons which innervate the auditory receptor cells form a spiral ganglion: a nerve-knot of the VIII pair’s acoustic part of the craniocerebral nerves. The ganglion fills the Rosental’s canal in the cochlea’s axis and re- peats the number of its spiral turns. The ganglionic neuron has, as a rule, a widened body with two processes: peripheral and central (Diagram 7). -

Auditory Neuropathy After Damage to Cochlear Spiral Ganglion Neurons
www.nature.com/scientificreports OPEN Auditory Neuropathy after Damage to Cochlear Spiral Ganglion Neurons in Mice Resulting from Conditional Received: 27 July 2016 Accepted: 15 June 2017 Expression of Diphtheria Toxin Published online: 25 July 2017 Receptors Haolai Pan1, Qiang Song1, Yanyan Huang1, Jiping Wang1, Renjie Chai2, Shankai Yin1 & Jian Wang 1,3 Auditory neuropathy (AN) is a hearing disorder characterized by normal cochlear amplifcation to sound but poor temporal processing and auditory perception in noisy backgrounds. These defcits likely result from impairments in auditory neural synchrony; such dyssynchrony of the neural responses has been linked to demyelination of auditory nerve fbers. However, no appropriate animal models are currently available that mimic this pathology. In this study, Cre-inducible diphtheria toxin receptor (iDTR+/+) mice were cross-mated with mice containing Cre (Bhlhb5-Cre+/−) specifc to spiral ganglion neurons (SGNs). In double-positive ofspring mice, the injection of diphtheria toxin (DT) led to a 30–40% rate of death for SGNs, but no hair cell damage. Demyelination types of pathologies were observed around the surviving SGNs and their fbers, many of which were distorted in shape. Correspondingly, a signifcant reduction in response synchrony to amplitude modulation was observed in this group of animals compared to the controls, which had a Cre− genotype. Taken together, our results suggest that SGN damage following the injection of DT in mice with Bhlhb5-Cre+/− and iDTR+/− is likely to be a good AN model of demyelination. Auditory neuropathy (AN) is a hearing disorder characterized as having normal cochlear microphonic (CM) potentials and otoacoustic emissions (OAEs), but largely reduced or missing auditory brainstem responses (ABRs). -

Cells of Adult Brain Germinal Zone Have Properties Akin to Hair Cells and Can Be Used to Replace Inner Ear Sensory Cells After Damage
Cells of adult brain germinal zone have properties akin to hair cells and can be used to replace inner ear sensory cells after damage Dongguang Weia,1, Snezana Levica, Liping Niea, Wei-qiang Gaob, Christine Petitc, Edward G. Jonesa, and Ebenezer N. Yamoaha,1 aDepartment of Anesthesiology and Pain Medicine, Center for Neuroscience, Program in Communication and Sensory Science, University of California, 1544 Newton Court, Davis, CA 95618; bDepartment of Molecular Biology, Genentech, Inc., South San Francisco, CA 94080; and cUnite´deGe´ne´ tique et Physiologie de l’Audition, Unite´Mixte de Recherche S587, Institut National de la Sante´et de la Recherche Me´dicale-Universite´Paris VI, Colle`ge de France, Institut Pasteur, 25 Rue du Dr Roux, 75724 Paris, Cedex 15, France Edited by David Julius, University of California, San Francisco, CA, and approved October 27, 2008 (received for review August 15, 2008) Auditory hair cell defect is a major cause of hearing impairment, often and have an actin-filled process as in the HCs. Thus, we surmise that leading to spiral ganglia neuron (SGN) degeneration. The cell loss that cells of the adult forebrain germinal zone might be potential follows is irreversible in mammals, because inner ear hair cells (HCs) candidate cells to be used autologously for the replacement of have a limited capacity to regenerate. Here, we report that in the nonrenewable HCs and SGNs. adult brain of both rodents and humans, the ependymal layer of the Ependymal cells adjacent to the spinal canal proliferate exten- lateral ventricle contains cells with proliferative potential, which sively upon spinal cord injuries (16, 17). -

Auditory and Vestibular Systems Objective • to Learn the Functional
Auditory and Vestibular Systems Objective • To learn the functional organization of the auditory and vestibular systems • To understand how one can use changes in auditory function following injury to localize the site of a lesion • To begin to learn the vestibular pathways, as a prelude to studying motor pathways controlling balance in a later lab. Ch 7 Key Figs: 7-1; 7-2; 7-4; 7-5 Clinical Case #2 Hearing loss and dizziness; CC4-1 Self evaluation • Be able to identify all structures listed in key terms and describe briefly their principal functions • Use neuroanatomy on the web to test your understanding ************************************************************************************** List of media F-5 Vestibular efferent connections The first order neurons of the vestibular system are bipolar cells whose cell bodies are located in the vestibular ganglion in the internal ear (NTA Fig. 7-3). The distal processes of these cells contact the receptor hair cells located within the ampulae of the semicircular canals and the utricle and saccule. The central processes of the bipolar cells constitute the vestibular portion of the vestibulocochlear (VIIIth cranial) nerve. Most of these primary vestibular afferents enter the ipsilateral brain stem inferior to the inferior cerebellar peduncle to terminate in the vestibular nuclear complex, which is located in the medulla and caudal pons. The vestibular nuclear complex (NTA Figs, 7-2, 7-3), which lies in the floor of the fourth ventricle, contains four nuclei: 1) the superior vestibular nucleus; 2) the inferior vestibular nucleus; 3) the lateral vestibular nucleus; and 4) the medial vestibular nucleus. Vestibular nuclei give rise to secondary fibers that project to the cerebellum, certain motor cranial nerve nuclei, the reticular formation, all spinal levels, and the thalamus. -

Smelling Better with Chloride COMMENTARY Stephan Fringsa,1
COMMENTARY Smelling better with chloride COMMENTARY Stephan Fringsa,1 The sense of smell and its astonishing performance coding is the solution to the problem of low-selectivity pose biologists with ever new riddles. How can the receptors (2). system smell almost anything that gets into the nose, However, the necessity to operate OSNs with fuzzy distinguish it from countless other odors, memorize odorant receptors creates another problem, as it limits it forever, and trigger reliably adequate behavior? the efficacy of the transduction process. OSNs trans- Among the senses, the olfactory system always duce chemical signals through a metabotropic path- seems to do things differently. The olfactory sensory way (Fig. 1A). Such pathways translate external stimuli neurons (OSNs) in the nose were suggested to use an into cellular responses by G-protein–coupled recep- unusual way of signal amplification to help them in tors. Their efficacy depends on the duration of recep- responding to weak stimuli. This chloride-based tor activity: the longer the receptor is switched on, the mechanism is somewhat enigmatic and controversial. more G protein can be activated. This is well studied in A team of sensory physiologists from The Johns photoreceptors, where the rhodopsin molecule may Hopkins University School of Medicine has now de- stay active for more than a second after absorbing a veloped a method to study this process in detail. photon. Within this time, it can activate hundreds of G Li et al. (1) demonstrate how OSNs amplify their proteins, one after the other, thus eliciting a robust electrical response to odor stimulation using chlo- cellular response to a single photon. -

Review of Hair Cell Synapse Defects in Sensorineural Hearing Impairment
Otology & Neurotology 34:995Y1004 Ó 2013, Otology & Neurotology, Inc. Review of Hair Cell Synapse Defects in Sensorineural Hearing Impairment *†‡Tobias Moser, *Friederike Predoehl, and §Arnold Starr *InnerEarLab, Department of Otolaryngology, University of Go¨ttingen Medical School; ÞSensory Research Center SFB 889, þBernstein Center for Computational Neuroscience, University of Go¨ttingen, Go¨ttingen, Germany; and §Department of Neurology, University of California, Irvine, California, U.S.A. Objective: To review new insights into the pathophysiology of are similar to those accompanying auditory neuropathy, a group sensorineural hearing impairment. Specifically, we address defects of genetic and acquired disorders of spiral ganglion neurons. of the ribbon synapses between inner hair cells and spiral ganglion Genetic auditory synaptopathies include alterations of glutamate neurons that cause auditory synaptopathy. loading of synaptic vesicles, synaptic Ca2+ influx or synaptic Data Sources and Study Selection: Here, we review original vesicle turnover. Acquired synaptopathies include noise-induced publications on the genetics, animal models, and molecular hearing loss because of excitotoxic synaptic damage and subse- mechanisms of hair cell ribbon synapses and their dysfunction. quent gradual neural degeneration. Alterations of ribbon synapses Conclusion: Hair cell ribbon synapses are highly specialized to likely also contribute to age-related hearing loss. Key Words: enable indefatigable sound encoding with utmost temporal precision. GeneticsVIon -

Cranial Nerve VIII
Cranial Nerve VIII Color Code Important (The Vestibulo-Cochlear Nerve) Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives At the end of the lecture, the students should be able to: ✓ List the nuclei related to vestibular and cochlear nerves in the brain stem. ✓ Describe the type and site of each nucleus. ✓ Describe the vestibular pathways and its main connections. ✓ Describe the auditory pathway and its main connections. Due to the difference of arrangement of the lecture between the girls and boys slides we will stick to the girls slides then summarize the pathway according to the boys slides. Ponto-medullary Sulcus (cerebello- pontine angle) Recall: both cranial nerves 8 and 7 emerge from the ventral surface of the brainstem at the ponto- medullary sulcus (cerebello-pontine angle) Brain – Ventral Surface Vestibulo-Cochlear (VIII) 8th Cranial Nerve o Type: Special sensory (SSA) o Conveys impulses from inner ear to nervous system. o Components: • Vestibular part: conveys impulses associated with body posture ,balance and coordination of head & eye movements. • Cochlear part: conveys impulses associated with hearing. o Vestibular & cochlear parts leave the ventral surface* of brain stem through the pontomedullary sulcus ‘at cerebellopontine angle*’ (lateral to facial nerve), run laterally in posterior cranial fossa and enter the internal acoustic meatus along with 7th (facial) nerve. *see the previous slide Auditory Pathway Only on the girls’ slides 04:14 Characteristics: o It is a multisynaptic pathway o There are several locations between medulla and the thalamus where axons may synapse and not all the fibers behave in the same manner. -
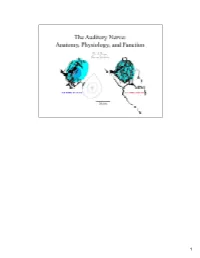
Auditory Nerve.Pdf
1 Sound waves from the auditory environment all combine in the ear canal to form a complex waveform. This waveform is deconstructed by the cochlea with respect to time, loudness, and frequency and neural signals representing these features are carried into the brain by the auditory nerve. It is thought that features of the sounds are processed centrally along parallel and hierarchical pathways where eventually percepts of the sounds are organized. 2 In mammals, the neural representation of acoustic information enters the brain by way of the auditory nerve. The auditory nerve terminates in the cochlear nucleus, and the cochlear nucleus in turn gives rise to multiple output projections that form separate but parallel limbs of the ascending auditory pathways. How the brain normally processes acoustic information will be heavily dependent upon the organization of auditory nerve input to the cochlear nucleus and on the nature of the different neural circuits that are established at this early stage. 3 This histology slide of a cat cochlea (right) illustrates the sensory receptors, the auditory nerve, and its target the cochlear nucleus. The orientation of the cut is illustrated by the pink line in the drawing of the cat head (left). We learned about the relationship between these structures by inserting a dye-filled micropipette into the auditory nerve and making small injections of the dye. After histological processing, stained single fibers were reconstruct back to their origin, and traced centrally to determine how they terminated in the brain. We will review the components of the nerve with respect to composition, innervation of the receptors, cell body morphology, myelination, and central terminations. -

Purinergic Signaling in Cochlear Supporting Cells Reduces Hair
RESEARCH ARTICLE Purinergic signaling in cochlear supporting cells reduces hair cell excitability by increasing the extracellular space Travis A Babola1, Calvin J Kersbergen1, Han Chin Wang1†, Dwight E Bergles1,2,3* 1The Solomon Snyder Department of Neuroscience, Johns Hopkins University, Baltimore, United States; 2Department of Otolaryngology Head and Neck Surgery, Johns Hopkins University, Baltimore, United States; 3Kavli Neuroscience Discovery Institute, Johns Hopkins University, Baltimore, United States Abstract Neurons in developing sensory pathways exhibit spontaneous bursts of electrical activity that are critical for survival, maturation and circuit refinement. In the auditory system, intrinsically generated activity arises within the cochlea, but the molecular mechanisms that initiate this activity remain poorly understood. We show that burst firing of mouse inner hair cells prior to hearing onset requires P2RY1 autoreceptors expressed by inner supporting cells. P2RY1 activation triggers K+ efflux and depolarization of hair cells, as well as osmotic shrinkage of supporting cells that dramatically increased the extracellular space and speed of K+ redistribution. Pharmacological inhibition or genetic disruption of P2RY1 suppressed neuronal burst firing by reducing K+ release, but unexpectedly enhanced their tonic firing, as water resorption by supporting cells reduced the extracellular space, leading to K+ accumulation. These studies indicate that purinergic signaling in *For correspondence: supporting cells regulates hair cell -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria.