Vaccine Use and Strategies for Elimination of Measles, Rubella, and Congenital Rubella Syndrome and Control of Mumps
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Communicable Disease Chart
COMMON INFECTIOUS ILLNESSES From birth to age 18 Disease, illness or organism Incubation period How is it spread? When is a child most contagious? When can a child return to the Report to county How to prevent spreading infection (management of conditions)*** (How long after childcare center or school? health department* contact does illness develop?) To prevent the spread of organisms associated with common infections, practice frequent hand hygiene, cover mouth and nose when coughing and sneezing, and stay up to date with immunizations. Bronchiolitis, bronchitis, Variable Contact with droplets from nose, eyes or Variable, often from the day before No restriction unless child has fever, NO common cold, croup, mouth of infected person; some viruses can symptoms begin to 5 days after onset or is too uncomfortable, fatigued ear infection, pneumonia, live on surfaces (toys, tissues, doorknobs) or ill to participate in activities sinus infection and most for several hours (center unable to accommodate sore throats (respiratory diseases child’s increased need for comfort caused by many different viruses and rest) and occasionally bacteria) Cold sore 2 days to 2 weeks Direct contact with infected lesions or oral While lesions are present When active lesions are no longer NO Avoid kissing and sharing drinks or utensils. (Herpes simplex virus) secretions (drooling, kissing, thumb sucking) present in children who do not have control of oral secretions (drooling); no exclusions for other children Conjunctivitis Variable, usually 24 to Highly contagious; -

Safety of Immunization During Pregnancy a Review of the Evidence
Safety of Immunization during Pregnancy A review of the evidence Global Advisory Committee on Vaccine Safety © World Health Organization 2014 All rights reserved. Publications of the World Health Organization are available on the WHO website (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications –whether for sale or for non-commercial distribution– should be addressed to WHO Press through the WHO website (www.who.int/about/licensing/copyright_form/en/index.html). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. -

Proquad, INN-Measles, Mumps, Rubella and Varicella Vaccine (Live)
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT ProQuad powder and solvent for suspension for injection ProQuad powder and solvent for suspension for injection in a pre-filled syringe Measles, mumps, rubella and varicella vaccine (live). 2. QUALITATIVE AND QUANTITATIVE COMPOSITION After reconstitution, one dose (0.5 mL) contains: 1 Measles virus Enders’ Edmonston strain (live, attenuated) ........ not less than 3.00 log10 TCID * 50 1 Mumps virus Jeryl Lynn™ (Level B) strain (live, attenuated) ... not less than 4.30 log10 TCID * 50 2 Rubella virus Wistar RA 27/3 strain (live, attenuated)............... not less than 3.00 log10 TCID * 50 3 Varicella virus Oka/Merck strain (live, attenuated) ................... not less than 3.99 log10 PFU** *50% tissue culture infectious dose **plaque-forming units (1) Produced in chick embryo cells. (2) Produced in human diploid lung (WI-38) fibroblasts. (3) Produced in human diploid (MRC-5) cells. The vaccine may contain traces of recombinant human albumin (rHA). This vaccine contains a trace amount of neomycin. See section 4.3. Excipient(s) with known effect The vaccine contains 16 milligrams of sorbitol per dose. See section 4.4. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Powder and solvent for suspension for injection Before reconstitution, the powder is a white to pale yellow compact crystalline cake and the solvent is a clear colourless liquid. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications ProQuad is indicated for simultaneous vaccination against measles, mumps, rubella and varicella in individuals from 12 months of age. ProQuad can be administered to individuals from 9 months of age under special circumstances (e.g., to conform with national vaccination schedules, outbreak situations, or travel to a region with high prevalence of measles; see sections 4.2, 4.4, and 5.1). -

Evaluation of the Tetracore Orthopox Biothreat® Antigen Detection Assay
Journal of Virological Methods 187 (2013) 37–42 Contents lists available at SciVerse ScienceDirect Journal of Virological Methods jou rnal homepage: www.elsevier.com/locate/jviromet ® Evaluation of the Tetracore Orthopox BioThreat antigen detection assay using laboratory grown orthopoxviruses and rash illness clinical specimens a,∗ b a a a Michael B. Townsend , Adam MacNeil , Mary G. Reynolds , Christine M. Hughes , Victoria A. Olson , a a Inger K. Damon , Kevin L. Karem a Centers for Disease Control and Prevention, Division of High-Consequence Pathogens and Pathology, Poxvirus and Rabies Branch, 1600 Clifton Road NE, Mailstop G-06, Atlanta, GA 30333, United States b Centers for Disease Control and Prevention, Global Immunization Division, CGH, 1600 Clifton Road NE, Mailstop G-06, Atlanta, GA 30333, United States a b s t r a c t ® Article history: The commercially available Orthopox BioThreat Alert assay for orthopoxvirus (OPV) detection is piloted. Received 5 December 2011 This antibody-based lateral-flow assay labels and captures OPV viral agents to detect their presence. Serial Received in revised form 23 August 2012 dilutions of cultured Vaccinia virus (VACV) and Monkeypox virus (MPXV) were used to evaluate the sensi- Accepted 30 August 2012 tivity of the Tetracore assay by visual and quantitative determinations; specificity was assessed using a Available online 5 September 2012 small but diverse set of diagnostically relevant blinded samples from viral lesions submitted for routine ® OPV diagnostic testing. The BioThreat Alert assay reproducibly detected samples at concentrations of Keywords: 7 6 10 pfu/ml for VACV and MPXV and positively identified samples containing 10 pfu/ml in 4 of 7 inde- Monkeypox Orthopoxvirus pendent experiments. -

Summary of the WHO Position on Rubella Vaccine- July 2020 This
Summary of the WHO position on Rubella Vaccine- July 2020 This document replaces the WHO position paper on rubella vaccine published in the Weekly Epidemiological Record in July 2011. It incorporates the most recent developments in the field of rubella vaccines to provide updated guidance on the introduction and use of rubella-containing vaccines (RCVs) in national immunization schedules. It specifically updates guidance on co- administration of rubella vaccine with yellow fever (YF) vaccine and updates data and the WHO position on the control and elimination of rubella. Background Rubella is of public health importance because of the teratogenic potential of infections acquired during pregnancy. Rubella virus is generally recognized as the most common infectious cause of birth defects, accounting for an estimated 100 000 infants born with congenital rubella syndrome (CRS) each year worldwide. Infection with rubella virus within 12 days of conception and early pregnancy (usually within the first 8–10 weeks) may result in miscarriage, fetal death or CRS. For the rest of the population, rubella is an acute, usually mild viral infection transmitted by respiratory droplets, which affects susceptible children and adults worldwide. A number of live, attenuated rubella vaccines are currently available. Most rubella vaccines are available in combination with other vaccine antigens such as measles, mumps and varicella (MR, MMR, MMRV, respectively) and also in a monovalent formulation. WHO position In light of the global burden of CRS, the proven efficacy, effectiveness and safety of RCVs and regional elimination goals, WHO recommends that countries introduce RCVs into their national immunization programmes. This recommendation includes the opportunity offered by accelerated measles elimination activities to achieve both goals. -

Seroprevalence of TORCH Infections in Children
Chattagram Maa-O-Shishu Hospital Medical College Journal Volume 17, Issue 1, January 2018 Original Article Seroprevalence of TORCH Infections in Children Sanjoy Kanti Biswas1* Abstract 2 Md. Badruddoza Background : The acronym “TORCH” was introduced to highlight a group of Nahid Sultana1 pathogens that cause a congenital and perinatal infections: Toxoplasma gondi, rubella virus, Cytomegalovirus (CMV) and Herpes Simplex Virus (HSV). These pathogens are often associated with congenital anomalies. Congenital malformations 1 Department of Microbiology have a direct impact on the family. This study was undertaken to detect the Chattagram Maa-O-Shishu Hospital Medical College Chittagong, Bangladesh. serological evidence of TORCH infections in children, by establishing the presence of specific IgM antibodies. Methods: During the period 1st June 2016 to 30th May 2 Department of Paediatrics 2017, 58 suspected TORCH infection cases were included from Paediatrics Chattagram Maa-O-Shishu Hospital Medical College Chittagong, Bangladesh. Department of CMOSH for TORCH antibody detection. The children were in the age of 0 day to 1 year with an average age of 3.3±2.59 months. The serum samples were tested forIgM and IgG antibodies against TORCH agents by using enzyme linked immunoassay method (ELISA). Results: Among the 58 children, seropositivity was found in 55 (94.82%) cases. Of the 55 seropositive cases serological evidence for combination of IgM and IgG with any one of the TORCH agents was detected in 25 (43.10%) and IgG alone was detected in 30 (51.72%) children. IgM/IgG antibody positivity to Toxoplasma, Rubella, CMV and HSV was 21(36.21%), 50(86.21%), 52(89.66%) and 8(13.79%) respectively. -

Measles Diagnostic Tool
Measles Prodrome and Clinical evolution E Fever (mild to moderate) E Cough E Coryza E Conjunctivitis E Fever spikes as high as 105ºF Koplik’s spots Koplik’s Spots E E Viral enanthem of measles Rash E Erythematous, maculopapular rash which begins on typically starting 1-2 days before the face (often at hairline and behind ears) then spreads to neck/ the rash. Appearance is similar to “grains of salt on a wet background” upper trunk and then to lower trunk and extremities. Evolution and may become less visible as the of rash 1-3 days. Palms and soles rarely involved. maculopapular rash develops. Rash INCUBATION PERIOD Fever, STARTS on face (hairline & cough/coryza/conjunctivitis behind ears), spreads to trunk, Average 8-12 days from exposure to onset (sensitivity to light) and then to thighs/ feet of prodrome symptoms 0 (average interval between exposure to onset rash 14 day [range 7-21 days]) -4 -3 -2 -1 1234 NOT INFECTIOUS higher fever (103°-104°) during this period rash fades in same sequence it appears INFECTIOUS 4 days before rash and 4 days after rash Not Measles Rubella Varicella cervical lymphadenopathy. Highly variable but (Aka German Measles) (Aka Chickenpox) Rash E often maculopapular with Clinical manifestations E Clinical manifestations E Generally mild illness with low- Mild prodrome of fever and malaise multiforme-like lesions and grade fever, malaise, and lymph- may occur one to two days before may resemble scarlet fever. adenopathy (commonly post- rash. Possible low-grade fever. Rash often associated with painful edema hands and feet. auricular and sub-occipital). -

Vaccine Information for PARENTS and CAREGIVERS
NATIONAL INSTITUTE FOR COMMUNICABLE DISEASES Division of the National Health Laboratory Service VACCINE INFOR MATION FOR PARENTS & CAREGIVERS First Edition November 2016 Editors-in-Chief Nkengafac Villyen Motaze (MD, MSc, PhD fellow), Melinda Suchard (MBBCh, FCPath (SA), MMed) Edited by: Cheryl Cohen, (MBBCh, FCPath (SA) Micro, DTM&H, MSc (Epi), Phd) Lee Baker, (Dip Pharm) Lucille Blumberg, (MBBCh, MMed (Micro) ID (SA) FFTM (RCPS, Glasgow) DTM&H DOH DCH) Published by: Ideas Wise and Wonderful (IWW) for National Institute for Communicable Diseases (NICD) First Edition: Copyright © 2016 Contributions by: Clement Adu-Gyamfi (BSc Hons, MSc), Jayendrie Thaver (BSc), Kerrigan McCarthy, (MBBCh, FCPath (SA), DTM&H, MPhil (Theol) Kirsten Redman (BSc Hons), Nishi Prabdial-Sing (PhD), Nonhlanhla Mbenenge (MBBCh, MMED) Philippa Hime (midwife), Vania Duxbury (BSc Hons), Wayne Howard (BSc Hons) Acknowledgments: Amayeza, Vaccine Information Centre (http://www.amayeza-info.co.za/) Centre for Communicable Diseases Fact Sheets (http://www.cdc.gov/vaccines/hcp/vis/) World Health Organization Fact Sheets (http://www.who.int/mediacentre/factsheets/en/) National Department of Health, South Africa (http://www.health.gov.za/) Disclaimer This book is intended as an educational tool only. Information may be subject to change as schedules or formulations are updated. Summarized and simplified information is presented in this booklet. For full prescribing information and contraindications for vaccinations, please consult individual package inserts. There has been -

Investigation of Poxvirus Host-Range and Gene Expression in Mammalian Cells
ABSTRACT Title: INVESTIGATION OF POXVIRUS HOST-RANGE AND GENE EXPRESSION IN MAMMALIAN CELLS. Jorge David Méndez Ríos, Doctor of Philosophy, 2014. Directed By: Dr. Bernard Moss, Chief, Laboratory of Viral Diseases, NIAID, NIH; Adjunct Professor Department of Cell Biology and Molecular Genetics, University of Maryland; Members of the Poxviridae family have been known as human pathogens for centuries. Their impact in society included several epidemics that decimated the population. In the last few centuries, Smallpox was of great concern that led to the development of our modern vaccines. The systematic study of Poxvirus host-range and immunogenicity provided the knowledge to translate those observations into practice. After the global vaccination campaign by the World Health Organization, Smallpox was the first infectious disease to be eradicated. Nevertheless, diseases such as Monkeypox, Molluscum contagiosum, new bioterrorist threads, and the use of poxviruses as vaccines or vectors provided the necessity to further understand the host-range from a molecular level. Here, we take advantage of the newly developed technologies such as 454 pyrosequencing and RNA-Seq to address previously unresolved questions for the field. First, we were able to identify the Erytrhomelagia-related poxvirus (ERPV) 25 years after its isolation in Hubei, China. Whole-genome sequencing and bioinformatics identified ERPV as an Ectromelia strain closely related to the Ectromelia Naval strain. Second, by using RNA-Seq, the first MOCV in vivo and in vitro transcriptome was generated. New tools have been developed to support future research and for this human pathogen. Finally, deep-sequencing and comparative genomes of several recombinant MVAs (rMVAs) in conjunction with classical virology allowed us to confirm several genes (O1, F5, C17, F11) association to plaque formation in mammalian cell lines. -
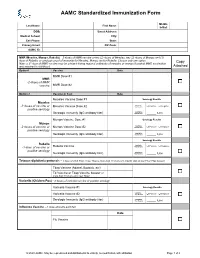
AAMC Standardized Immunization Form
AAMC Standardized Immunization Form Middle Last Name: First Name: Initial: DOB: Street Address: Medical School: City: Cell Phone: State: Primary Email: ZIP Code: AAMC ID: MMR (Measles, Mumps, Rubella) – 2 doses of MMR vaccine or two (2) doses of Measles, two (2) doses of Mumps and (1) dose of Rubella; or serologic proof of immunity for Measles, Mumps and/or Rubella. Choose only one option. Copy Note: a 3rd dose of MMR vaccine may be advised during regional outbreaks of measles or mumps if original MMR vaccination was received in childhood. Attached Option1 Vaccine Date MMR Dose #1 MMR -2 doses of MMR vaccine MMR Dose #2 Option 2 Vaccine or Test Date Measles Vaccine Dose #1 Serology Results Measles Qualitative -2 doses of vaccine or Measles Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Mumps Vaccine Dose #1 Serology Results Mumps Qualitative -2 doses of vaccine or Mumps Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Serology Results Rubella Qualitative Positive Negative -1 dose of vaccine or Rubella Vaccine Titer Results: positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Tetanus-diphtheria-pertussis – 1 dose of adult Tdap; if last Tdap is more than 10 years old, provide date of last Td or Tdap booster Tdap Vaccine (Adacel, Boostrix, etc) Td Vaccine or Tdap Vaccine booster (if more than 10 years since last Tdap) Varicella (Chicken Pox) - 2 doses of varicella vaccine or positive serology Varicella Vaccine #1 Serology Results Qualitative Varicella Vaccine #2 Titer Results: Positive Negative Serologic Immunity (IgG antibody titer) Quantitative Titer Results: _____ IU/ml Influenza Vaccine --1 dose annually each fall Date Flu Vaccine © 2020 AAMC. -
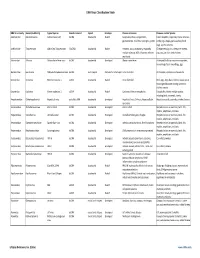
Virus Classification Tables V2.Vd.Xlsx
DNA Virus Classification Table DNA Virus Family Genera (Subfamily) Typical Species Genetic material Capsid Envelope Disease in Humans Diseases in other Species Adenoviridae Mastadenovirus Adenoviruses 1‐47 dsDNA Icosahedral Naked Respiratory illness; conjunctivitis, Canine hepatitis, respiratory illness in horses, gastroenteritis, tonsillitis, meningitis, cystitis cattle, pigs, sheep, goats, sea lions, birds dogs, squirrel enteritis Anelloviridae Torqueviruses Alpha‐Zeta Torqueviruses (‐)ssDNA Icosahedral Naked Hepatitis, lupus, pulmonary, myopathy, Chimpanzee, pig, cow, sheep, tree shrews, multiple sclerosis; 90% of humans infected pigs, cats, sea lions and chickens worldwide Asfarviridae Asfivirus African Swine fever virus dsDNA Icosahedral Enveloped African swine fever Arthropod (tick) transmission or ingestion; hemorrhagic fever in warthogs, pigs Baculoviridae Baculovirus Alpha‐Gamma Baculoviruses dsDNA Stick shaped Occluded or Enveloped none identified Arthropods, Lepidoptera, crustaceans Circoviridae Circovirus Porcine circovirus 1 ssDNA Icosahedral Naked none identified Birds, pigs, dogs; bats; rodents; causes post‐ weaning multisystem wasting syndrome, chicken anemia Circoviridae Cyclovirus Human cyclovirus 1 ssDNA Icosahedral Naked Cyclovirus Vietnam encephalitis Encephalitis; infects multiple species including birds, mammals, insects Hepadnaviridae Orthohepadnavirus Hepatitis B virus partially ssDNA Icosahedral Enveloped Hepatitis B virus; Cirrhosis, Hepatocellular Hepatitis in ducks, squirrels, primates, herons carcinoma Herpesviridae -

Recommended Adult Immunization Schedule
Recommended Adult Immunization Schedule UNITED STATES for ages 19 years or older 2021 Recommended by the Advisory Committee on Immunization Practices How to use the adult immunization schedule (www.cdc.gov/vaccines/acip) and approved by the Centers for Disease Determine recommended Assess need for additional Review vaccine types, Control and Prevention (www.cdc.gov), American College of Physicians 1 vaccinations by age 2 recommended vaccinations 3 frequencies, and intervals (www.acponline.org), American Academy of Family Physicians (www.aafp. (Table 1) by medical condition and and considerations for org), American College of Obstetricians and Gynecologists (www.acog.org), other indications (Table 2) special situations (Notes) American College of Nurse-Midwives (www.midwife.org), and American Academy of Physician Assistants (www.aapa.org). Vaccines in the Adult Immunization Schedule* Report y Vaccines Abbreviations Trade names Suspected cases of reportable vaccine-preventable diseases or outbreaks to the local or state health department Haemophilus influenzae type b vaccine Hib ActHIB® y Clinically significant postvaccination reactions to the Vaccine Adverse Event Hiberix® Reporting System at www.vaers.hhs.gov or 800-822-7967 PedvaxHIB® Hepatitis A vaccine HepA Havrix® Injury claims Vaqta® All vaccines included in the adult immunization schedule except pneumococcal 23-valent polysaccharide (PPSV23) and zoster (RZV) vaccines are covered by the Hepatitis A and hepatitis B vaccine HepA-HepB Twinrix® Vaccine Injury Compensation Program. Information on how to file a vaccine injury Hepatitis B vaccine HepB Engerix-B® claim is available at www.hrsa.gov/vaccinecompensation. Recombivax HB® Heplisav-B® Questions or comments Contact www.cdc.gov/cdc-info or 800-CDC-INFO (800-232-4636), in English or Human papillomavirus vaccine HPV Gardasil 9® Spanish, 8 a.m.–8 p.m.