La Ronge Northerner Articles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

True North // September 2017
True North // September 2017 cameco in northern saskatchewan Cameco partners with the Red Cross to support Pelican Narrows evacuees (p.2) WINTER Surviving off 2015 Land and Water Fond du Lac Canoe Quest is a Success Far From Home Red Cross and Cameco employees delivered baby strollers to young families from northeastern Saskatchewan while they were evacuated to Prince Albert and Saskatoon during the wildfires earlier this fall. “Once again, Cameco came through to help those Cameco proud to evacuated in northern Saskatchewan,” said Cindy support evacuees Fuchs, Vice-President of the Canadian Red Cross in during fires Saskatchewan. “We are so thankful for Cameco’s support – it makes a world of difference for people forced from their homes.” Wildfires forced more than 2,700 people from the Cree communities of Pelican Narrows and Sandy Bay in late August. The evacuation ban was lifted September 13. During that time evacuees stayed in Prince Albert and Saskatoon with the aid of the Red Cross. Cameco was proud to partner with the organization and provided baby strollers, movie passes and food to make the stay more comfortable. Cameco also contributed $25,000 to the Red Cross’s Red Gala. Proceeds from the gala help support disaster relief. source: Government of Saskatchewan Facebook page page 2 True North // September 2017 Fond du Lac Youth Canoe Quest imparts important traditional skills The participants in the Fond du Lac also visited the basecamp to perform, Toutsaint says the experience made Canoe Quest met with stunning as well as other members who wanted such an impression that the community sunrises for five days at the beginning to cheer the group along. -

Politics, Power, and Environmental Governance: a Comparative Case Study of Three Métis Communities in Northwest Saskatchewan
University of Alberta Politics, Power, and Environmental Governance: A Comparative Case Study of Three Métis Communities in Northwest Saskatchewan by Bryn Alan Politylo A thesis submitted to the Faculty of Graduate Studies and Research in partial fulfillment of the requirements for the degree of Master of Science in Rural Sociology Department of Resource Economics and Environmental Sociology ©Bryn Alan Politylo Fall 2011 Edmonton, Alberta Permission is hereby granted to the University of Alberta Libraries to reproduce single copies of this thesis and to lend or sell such copies for private, scholarly or scientific research purposes only. Where the thesis is converted to, or otherwise made available in digital form, the University of Alberta will advise potential users of the thesis of these terms. The author reserves all other publication and other rights in association with the copyright in the thesis and, except as herein before provided, neither the thesis nor any substantial portion thereof may be printed or otherwise reproduced in any material form whatsoever without the author's prior written permission. Abstract Recently northwest Saskatchewan has seen a rapid push towards large-scale development corresponding with a shifting political economy in the province. For the rights- bearing Métis people of northwest Saskatchewan this shift significantly influences provincial environmental governance, which affects the agency of Métis people to participate in natural resource management and decision-making in the region. To examine the agency and power of Métis communities in provincial natural resource management and decision-making, qualitative methods and a comparative case study of three Métis communities were used to analyze and interpret the social spaces that Métis people occupy in provincial environmental governance. -

Northern Saskatchewan Administration District (NSAD)
Northern Saskatchewan Administration District (NSAD) Camsell Uranium ´ Portage City Stony Lake Athasbasca Rapids Athabasca Sand Dunes Provincial Park Cluff Lake Points Wollaston North Eagle Point Lake Airport McLean Uranium Mine Lake Cigar Lake Uranium Rabbit Lake Wollaston Mine Uranium Mine Lake McArthur River 955 Cree Lake Key Lake Uranium Reindeer Descharme Mine Lake Lake 905 Clearwater River Provincial Park Turnor 914 La Loche Lake Garson Black Lake Point Bear Creek Southend Michel Village St. Brabant George's Buffalo Hill Patuanak Narrows 102 Seabee 155 Gold Mine Santoy Missinipe Lake Gold Sandy Ile-a-la-crosse Pinehouse Bay Stanley Mission Wadin Little Bay Pelican Amyot Lac La Ronge Jans Bay La Plonge Provincial Park Narrows Cole Bay 165 La Ronge Beauval Air Napatak Keeley Ronge Tyrrell Lake Jan Lake Lake 55 Sturgeon-Weir Creighton Michel 2 Callinan Point 165 Dore Denare Lake Tower Meadow Lake Provincial Park Beach Beach 106 969 916 Ramsey Green Bay Weyakwin East 55 Sled Trout Lake Lake 924 Lake Little 2 Bear Lake 55 Prince Albert Timber National Park Bay Prince Albert Whelan Cumberland Little Bay Narrow Hills " Peck Fishing G X Delaronde National Park Provincial Park House NortLahke rLnak eTowns Northern Hamlets ...Northern Settlements 123 Creighton Black Point Descharme Lake 120 Noble's La Ronge Cole Bay Garson Lake 2 Point Dore Lake Missinipe # Jans Bay Sled Lake Ravendale Northern Villages ! Peat Bog Michel Village Southend ...Resort Subdivisions 55 Air Ronge Patuanak Stanley Mission Michel Point Beaval St. George's Hill Uranium -

Community Investment in the Pandemic: Trends and Opportunities
Community investment in the pandemic: trends and opportunities Jonathan Huntington, Vice President Sustainability and Stakeholder Relations, Cameco January 6, 2021 A Cameco Safety Moment Recommended for the beginning of any meeting Community investment in the pandemic: trends and opportunities (January 6, 2021) 2 Community investment in the pandemic: Trends • Demand - increase in requests • $1 million Cameco COVID Relief Fund: 581 applications, $17.5 million in requests • Immense competition for funding dollars • We supported 67 community projects across 40 different communities in SK Community investment in the pandemic: trends and opportunities (January 6, 2021) 3 Successful applicants for Cameco COVID Relief Fund Organization Community Organization Community Children North Family Resource Center La Ronge The Generation Love Project Saskatoon Prince Albert Child Care Co-operative Association Prince Albert Lakeview Extended School Day Program Inc. Saskatoon Central Urban Metis Federation Inc. Saskatoon Delisle Elementary School -Hampers Delisle TLC Daycare Inc. Birch Hills English River First Nation English River Beauval Group Home (Shirley's Place) Beauval NorthSask Special Needs La Ronge Nipawin Daycare Cooperative Nipawin Leask Community School Leask Battlefords Interval House North Battleford Metis Central Western Region II Prince Albert Beauval Emergency Operations - Incident Command Beauval Global Gathering Place Saskatoon Northern Hamlet of Patuanak Patuanak Saskatoon YMCA Saskatoon Northern Settlement of Uranium City Uranium City -

Cameco COVID-19 Relief Fund Supports 67 Community Projects
TSX: CCO website: cameco.com NYSE: CCJ currency: Cdn (unless noted) 2121 – 11th Street West, Saskatoon, Saskatchewan, S7M 1J3 Canada Tel: 306-956-6200 Fax: 306-956-6201 Cameco COVID-19 Relief Fund Supports 67 Community Projects Saskatoon, Saskatchewan, Canada, April 30, 2020 . Cameco (TSX: CCO; NYSE: CCJ) is pleased to announce that the company is supporting 67 community projects in Saskatoon and northern Saskatchewan through its $1 million Cameco COVID-19 Relief Fund. “There are so many communities and charitable groups hit hard by this pandemic, yet their services are needed now more than ever,” said Cameco president and CEO Tim Gitzel. “We are extremely happy to be able to help 67 of these organizations continue to do the vital work they do every day to keep people safe and supported through this unprecedented time.” Approved projects come from 40 Saskatchewan communities from Saskatoon to the province’s far north. A full listing can be found at the end of this release. Included in the support Cameco is providing are significant numbers of personal protective equipment (PPE) for northern Saskatchewan communities and First Nations – 10,000 masks, 7,000 pairs of gloves and 7,000 litres of hand sanitizer. Donations of supplies and money from nearly 100 Cameco employees augmented the company’s initial $1 million contribution. Cameco will move quickly to begin delivering this support to the successful applicants. “I’m proud of Cameco’s employees for stepping up yet again to support the communities where they live,” Gitzel said. “It happens every time we put out a call for help, a call for volunteers, a call to assist with any of our giving campaigns, and I can’t say enough about their generosity.” Announced on April 15, the Cameco COVID-19 Relief Fund was open to applications from charities, not-for-profit organizations, town offices and First Nation band offices in Saskatoon and northern Saskatchewan that have been impacted by the pandemic. -

Business Directory Listings Prepared May 14/2021
Business Directory Listings Prepared May 14/2021 A & A Logging Amachewespemawin Co-operative Assoc. Ltd. Box 157 Box 250 Green Lake, SK Stanley Mission, SK S0M 1B0 S0J 2P0 Location: Green Lake Location: Stanley Mission Contact: Art Laliberte Contact: Eva McKenzie, Acting Manager Tel: 306-832-2100 Tel: 306-635-2020 Fax: 306-832-2100 Fax: 306-635-2070 Description: Stump to dump & log hauling. Email: [email protected] Description: Retail store, Gas Bar, & Groceries. A & L Transport Box 155 Amachewespimawin Co-operative Restaurant Stony Rapids, SK Box 250 S0J 2R0 Stanley Mission, SK Location: Stony Rapids S0J 2P0 Contact: Morris Gabrush, Owner Location: Stanley Mission Tel: 306-439-2157 Contact: Pam McLeod, Manager Fax: 306-439-4992 Tel: 306-635-2093 Email: [email protected] Fax: 306-635-2070 Description: Trucking, Storage, Light Truck Rental Description: Chester Fried Chicken, fast food Units, Construction Equipment: Dozer, restaurant. Loader, Motor Grader, Rick Truck, Fuel Truck, Water Truck, Tractor/Skidder and Gravel Screener Amys Bar & Grill Motel Beauval, SK S0M 0G0 Aboriginal Headstart Location: Beauval Box 269 Contact: Mitch Beauval, SK Tel: 306-288-4700 S0M 0G0 Description: 7 room motel (satellite), Tavern & Off Location: Beauval Sale. Contact: Patty Gauthier Tel: 306-288-2274 Fax: 306-288-4502 Andys Store Email: [email protected] Box 58 Description: School for 3 & 4 year olds & parental Southend, SK support. S0J 2L0 Location: Southend Contact: Andy Park Als Place Motel Tel: 306-758-0001 Box 126 Fax: 306-758-0002 Stony Rapids, SK Description: Gas, diesel, grocery, confectionery, & S0J 2R0 ATM. Location: Stony Rapids Contact: Al Sayn Tel: 306-439-2057 Fax: 306-439-2047 Email: [email protected] Website: www.alsplace.ca Description: Deluxe guest rooms,restaurant. -
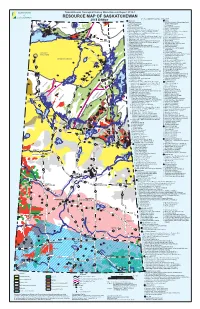
Mineral Resource Map of Saskatchewan
Saskatchewan Geological Survey Miscellaneous Report 2018-1 RESOURCE MAP OF SASKATCHEWAN KEY TO NUMBERED MINERAL DEPOSITS† 2018 Edition # URANIUM # GOLD NOLAN # # 1. Laird Island prospect 1. Box mine (closed), Athona deposit and Tazin Lake 1 Scott 4 2. Nesbitt Lake prospect Frontier Adit prospect # 2 Lake 3. 2. ELA prospect TALTSON 1 # Arty Lake deposit 2# 4. Pitch-ore mine (closed) 3. Pine Channel prospects # #3 3 TRAIN ZEMLAK 1 7 6 # DODGE ENNADAI 5. Beta Gamma mine (closed) 4. Nirdac Creek prospect 5# # #2 4# # # 8 4# 6. Eldorado HAB mine (closed) and Baska prospect 5. Ithingo Lake deposit # # # 9 BEAVERLODGE 7. 6. Twin Zone and Wedge Lake deposits URANIUM 11 # # # 6 Eldorado Eagle mine (closed) and ABC deposit CITY 13 #19# 8. National Explorations and Eldorado Dubyna mines 7. Golden Heart deposit # 15# 12 ### # 5 22 18 16 # TANTATO # (closed) and Strike deposit 8. EP and Komis mines (closed) 14 1 20 #23 # 10 1 4# 24 # 9. Eldorado Verna, Ace-Fay, Nesbitt Labine (Eagle-Ace) 9. Corner Lake deposit 2 # 5 26 # 10. Tower East and Memorial deposits 17 # ###3 # 25 and Beaverlodge mines and Bolger open pit (closed) Lake Athabasca 21 3 2 10. Martin Lake mine (closed) 11. Birch Crossing deposits Fond du Lac # Black STONY Lake 11. Rix-Athabasca, Smitty, Leonard, Cinch and Cayzor 12. Jojay deposit RAPIDS MUDJATIK Athabasca mines (closed); St. Michael prospect 13. Star Lake mine (closed) # 27 53 12. Lorado mine (closed) 14. Jolu and Decade mines (closed) 13. Black Bay/Murmac Bay mine (closed) 15. Jasper mine (closed) Fond du Lac River 14. -

12Th Annual Report for the Period April 1St, 2013 to March 31St, 2014 Compiled by Michael Fulton, Educon Services Inc
KidsFirst NORTH Staff at August 2013 Retreat in Saskatoon 12th Annual Report For the period April 1st, 2013 to March 31st, 2014 Compiled by Michael Fulton, Educon Services Inc. Table of Contents Program Manager’s Foreword 3 Acknowledgements 4 Introduction and Background Information 6 Vision, Model Description and Guiding Principles Organization Chart and Personnel Goals and Accomplishments Challenges Health Regions, Communities Served and Population Home Visiting Supervision, Home Visitor and Program Support Summaries Agency Contracts and Services Significant Program Enhancements in 2013-14 Prenatal Families and Screening and Assessment Data Regional Reports 18 MCRRHA-Creighton/Denare Beach, Sandy Bay, La Ronge, Air Ronge and Pinehouse KYRHA-Buffalo Narrows, La Loche, Beauval, Green Lake and Ile-a-la-Crosse KTRHA-Cumberland House AHA-The Far North including Stony Rapids Community Development Report 27 Mental Health Reports 35 MCRRHA Region-Penny Frazer KYRHA Region-Dawnali Reimer Professional Development and Staff Training Report 40 Good News Stories from the Regions 49 Parenting and Family Supports Early Childhood Development and Learning Mental Health and Healthy Lifestyles Community Supports Program Manager’s Concluding Remarks 75 KidsFirst NORTH Addresses and Contact Information 76 2 Program Manager’s Foreword KidsFirst NORTH celebrates its 12th birthday this year. Looking back on the work we have done this past year we see that our focus on relationships, strengthening parents and family units and developing our program in our beautiful Northern Communities have been central to our work to growing great children and families. We are proud to serve the families in Northern Saskatchewan and love helping our families to be safe, secure, happy and healthy. -

18-166-028 Ccotruenorth Sept-Insert PRINT
Energizing the Fond du Lac future through 2018 education Northern Saskatchewan Scholarship Recipients La Loche Buffalo Narrows Pinehouse Ile a la Crosse La Ronge Pelican Narrows Air Ronge Deschambeault Lake Denare Beach Cameco is committed to making a positive, long-term difference in local communities. When Cameco announced a new northern Saskatchewan scholarship in March this year, we expected a lot of applications. With more than 70 bright, young applicants from all across Saskatchewan’s north, our expectations were surpassed. We’re delighted to help 15 “ I love my community and Cameco is committed to making a students begin their new school would love to be a part positive, long-term difference in local year in post-secondary institutions of helping it thrive.” communities. The Cameco Northern in Saskatchewan. Their fields of study Saskatchewan Scholarship “ Upon completion I plan is one are as diverse as they are and this to teach in northern of the many ways in which we do so. year’s recipients come from all across Saskatchewan. I would like Based on the goals they’ve described, the north. Many of this year’s recipients to teach the youth about it’s apparent that many of these have expressed a goal of using their communities and our culture.” students are equally committed education and degrees to contribute to making a positive difference to their home community: “WhenIamfinishedmypost- back in their home communities. secondary education, my goal Congratulations to each of you; is to move back to my hometown we wish you every success and help the Métis Nation in your studies. -

Recent Ethnographic Research—Upper Churchill River Drainage, Saskatchewan, Canada
ARCTlC VOL. 32, NO. 4 (DEC. 1979), P. 355-365 Recent EthnographicResearch-- Upper Churchill River Drainage, Saskatchewan, Canada ROBERT JARVENPA’ ABSTRACT. Recent developments in ethnographic research in the Upper Churchill River drainage of northwestern Saskatchewan are reviewed. These include an analysis of the spatial organization of trapping economics, and an examination of behavioral responses to current technological impact (particularly housing, imported food and machinery, and new roads) ain southern Chipewyan community. Although high-income trappers generally exploit the largest trapping areasat the greatest distances from a primary settlement, the increasing congregation of short-distance trappersnear the villagemay be exacerbating ecological and economic instability associated with new consumer goods and purchasing habits. Another direction of research involves analysis of economic and social interactions between Chipewyan and Cree communities that shed lightupon processes of inter-tribal communication, symbiosis, enmity and identity management. INTRODUCTION During the summer field seasons of 1975 and 1977 faculty and graduate students from the State University of New York at Albany Department of Anthropology, in conjunction with the Urgent Ethnology Programme of the National Museums of Canada, conducted team research projects focusing on changing ecological and cultural conditions in Chipewyan and Cree Indian communities in the Upper Churchill River drainage of northwestern Saskatchewan (Fig. 1). Spatial Mobility and Subarctic Trapping Economies The regionally-based team investigations of recent years are an outgrowth of an earlier study of the ecology and spatial organization of the Chipewyan community of Patuanak,Saskatchewan (Jarvenpa, 1975). Thelatter work, based upon research conducted during 1971-72, emphasizes the geographical mobility of commercial fur trappers and fishermen as a variable forexplaining the organization of economic-subsistence cycles and ongoing processes of settlement pattern change (Fig. -

Business Directory Prepared July 12/2016
Pinehouse Community Business Directory Prepared July 12/2016 Annie Johnston - Awasis Centre Jonlaw Development Corporation Box 89 Pinehouse Pinehouse, SK Location: Pinehouse S0J 2B0 Contact: Jon Location: Pinehouse Tel: 306-884-4981 Contact: Shannon Natomagan Fax: 306-884-2341 Tel: 306-884-4868 Email: [email protected] Fax: 306-884-4818 Description: Nightly, weekly, & monthly living Description: Programming for preschool children. quarters. Beaver River Regional Housing Authority Kamkota Lodge Box 395 Box 225 Pinehouse, SK Pinehouse, SK S0J 2B0 S0J 2B0 Location: Pinehouse Location: Pinehouse Contact: Bernice La Riviere Contact: Kamilla Ukrainnetz Tel: 306-884-2038 Tel: 306-884-2177 Fax: 306-884-2039 Fax: 306-884-2187 Email: [email protected] Email: [email protected] Description: Rental of remote housing & maintenance. Website: www.kamkotalodge.com Description: Hunting & fishing camp-seasonal, accommodations, boat & motor rental, Cameco Northern Affairs Office - Pinehouse guiding-full services available. IR General Delivery Pinehouse, SK Kids First North Program Location: Pinehouse Location: Pinehouse Contact: Denise Natamogan Tel: 306-884-2117 Tel: 306-884-2357 Fax: 306-884-2108 Fax: 306-884-2359 Description: Home Visits To Assist In Parenting. Email: [email protected] Kineepik Metis Local Inc # 9 Box 166 CFNK Radio - Minahik Achimowin Inc. Pinehouse Box 370 Location: Pinehouse Pinehouse Tel: 306-884-2016 Location: Pinehouse Fax: 306-884-2365 Contact: Vince Natamagan, Manager Email: [email protected] Tel: 306-884-2011 -

View: the Following Is a List of Programs and Their Functional Areas of Responsibility
Mamawetan Churchill River Health Region 2004-2005 Annual Report Mamawetan Churchill River Regional Health Authority 2004-2005 Annual Report BOARD OF DIRECTORS Louise Wiens, Chairperson - La Ronge (306) 425-2119 Mary Denechezhe, Vice-Chairperson - Wollaston Lake (306) 633-4849 William Dumais - Southend (306) 758- 2154 Charlene Logan - Flin Flon / Creighton / Denare Beach (306) 688-7437 Al Rivard - La Ronge (306) 425-3961 Tammy Cook-Searson - La Ronge Lac La Ronge Indian Band (306) 425-5000 Peter Bear – Sandy Bay (306) 754-2165 Ida Ratt-Natomagan – Pinehouse (306) 425-4860 Ron Woytowich – La Ronge (306) 425-2051 Al Loke – La Ronge (306) 425-5505 Larry Beatty – Deschambault Lake (306) 632-2106 Together in Wellness Page 2 of 70 Mamawetan Churchill River Regional Health Authority 2004-2005 Annual Report Table of Contents GLOSSARY OF ABBREVIATIONS ......................................................................................................... 6 WHO WE ARE............................................................................................................................................. 8 MISSION, VISION AND VALUES:................................................................................................................ 8 GOVERNANCE AND ORGANIZATION:...................................................................................................... 10 HEALTH CARE ORGANIZATIONS & OTHER THIRD PARTY RELATIONSHIPS:...................................... 12 REGIONAL ENVIRONMENTAL SCAN:.............................................................................................