Estradiol (Topical Use) Art 31
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Postmenopausal Pharmacotherapy Newsletter
POSTMENOPAUSAL PHARMACOTHERAPY September, 1999 As Canada's baby boomers age, more and more women will face the option of Hormone Replacement Therapy (HRT). The HIGHLIGHTS decision can be a difficult one given the conflicting pros and cons. M This RxFiles examines the role and use of HRT, as well as newer Long term HRT carries several major benefits but also risks SERMS and bisphosphonates in post-menopausal (PM) patients. which should be evaluated on an individual and ongoing basis MContinuous ERT is appropriate for women without a uterus HRT MWomen with a uterus should receive progestagen (at least 12 HRT is indicated for the treatment of PM symptoms such as days per month or continuous low-dose) as part of their HRT vasomotor disturbances and urogenital atrophy, and is considered MLow-dose ERT (CEE 0.3mg) + Ca++ appears to prevent PMO primary therapy for prevention and treatment of postmenopausal MBisphosphinates (e.g. alendronate, etidronate) and raloxifene are osteoporosis (PMO).1 Contraindications are reviewed in Table 2. alternatives to HRT in treating and preventing PMO Although HRT is contraindicated in women with active breast or M"Natural" HRT regimens can be compounded but data is lacking uterine cancer, note that a prior or positive family history of these does not necessarily preclude women from receiving HRT.1 Comparative Safety: Because of differences between products, some side effects may be alleviated by switching from one product Estrogen Replacement Therapy (ERT) 2 to another, particularly from equine to plant sources or from oral to Naturally secreted estrogens include: topical (see Table 3 - Side Effects & Their Management). -

Estrogen Agents, Oral-Transdermal
GEORGIA MEDICAID FEE-FOR-SERVICE ESTROGEN AGENTS, ORAL - TRANSDERMAL PA SUMMARY Preferred Non-Preferred Oral Estrogens Estradiol generic n/a Menest (esterified estrogens) Premarin (estrogens, conjugated) Oral Estrogen/Progestin Combinations Angeliq (drospirenone/estradiol) Bijuva (estradiol/progesterone) Estradiol/norethindrone and all generics for Activella Norethindrone/ethinyl estradiol and all generics for Femhrt Low Dose 0.5/2.5 (norethindrone/ethinyl Femhrt Low Dose estradiol) Jinteli and all generics for Femhrt 1/5 (norethindrone/ethinyl estradiol) Prefest (estradiol/norgestimate) Premphase (conjugated estrogens/medroxyprogesterone) Prempro (conjugated estrogens/medroxyprogesterone) Topical Estrogens Alora (estradiol transdermal patch) Divigel (estradiol topical gel) Estradiol transdermal patch (generic Climara) Elestrin (estradiol topical gel) Evamist (estradiol topical spray solution) Estradiol transdermal patch (generic Vivelle-Dot) Menostar (estradiol transdermal patch) Minivelle (estradiol transdermal patch) Vivelle-Dot (estradiol transdermal patch) Topical Estrogens/Progestin Combination Climara Pro (estradiol/levonorgestrel transdermal patch) n/a Combipatch (estradiol/norethindrone transdermal patch) Oral Selective Estrogen Receptor Modulator (SERMs) Raloxifene generic Duavee (conjugated estrogens/bazedoxifene) Osphena (ospemifene) LENGTH OF AUTHORIZATION: 1 year PA CRITERIA: Bijuva ❖ Approvable for the treatment of moderate to severe vasomotor symptoms associated with menopause in women with an intact uterus who have experienced inadequate response, allergies, contraindications, drug-drug interactions or intolerable side effects to at least two preferred oral estrogen/progestin combination products. Revised 6/29/2020 Norethindrone/Ethinyl Estradiol and All Generics for Femhrt Low Dose ❖ Prescriber must submit a written letter of medical necessity stating the reasons at least two preferred oral estrogen/progestin combination products, one of which must be brand Femhrt Low Dose, are not appropriate for the member. -

Why the Product Labeling for Low-Dose Vaginal Estrogen Should Be Changed
Menopause: The Journal of The North American Menopause Society Vol. 21, No. 9, pp. 911/916 DOI: 10.1097/gme.0000000000000316 * 2014 by The North American Menopause Society EDITORIAL Why the product labeling for low-dose vaginal estrogen should be changed his commentary summarizes the activities of several of a highly effective local treatment of a common condition clinicians and researchers to encourage modifications with medical risks and adverse effects on quality of life. We Tto the labeling of low-dose vaginal estrogen. Motivated contend that the boxed warning for low-dose vaginal es- by concerns of practicing clinicians that the boxed warning on trogen products is unjustified based on several lines of rea- the labels and package inserts for these products overstate po- soning described below, including the following: (a) the tential risks and thus adversely affect patient care, leaders dramatic differences in blood hormone levels achieved by in the field are spearheading an effort to encourage consider- low-dose vaginal estrogen (eg, Vagifem tablets [estradiol ation of alternative labeling, as discussed below. The members 10 Kg], Estring [releasing estradiol 7.5 Kg/d], or comparable of the Working Group on Women’s Health and Well-Being in low-dose vaginal estrogen cream formulations) versus con- Menopause have affiliations with a number of medical socie- ventional systemic estrogen therapy; (b) absence of ran- ties, including The North American Menopause Society, the domized trial evidence or consistent observational evidence American College of Obstetricians and Gynecologists, the En- linking low-dose vaginal estrogen to cancer, cardiovascular docrine Society, the American Society for Reproductive Med- disease, dementia, or any of the other conditions highlighted icine, the International Society for the Study of Women’s in the boxed warning; and (c) absence of evidence that changes in Sexual Health, and other professional organizations. -

Chronic Unopposed Vaginal Estrogen Therapy
October, 1999. The Rx Files: Q&A Summary S. Downey BSP, L.D. Regier BSP, BA Chronic Unopposed Vaginal Estrogen Therapy The question of whether progestagen opposition is required in a patient on chronic vaginal estrogen is controversial. The literature is not clear on this matter and the SOGC conference on Menopause did not reach a consensus. Endometrial hyperplasia is directly related to the dose and duration of estrogen therapy. The PEPI study showed that 10 per cent of women taking unopposed estrogen (equivalent to 0.625 mg CEE) will develop complex or atypical endometrial hyperplasia within 1 year. With long-term HRT, it is now considered standard practice to add progestagen opposition to oral estrogen therapy in women with an intact uterus. The case is less clear for vaginal estrogen therapy. The makers of Premarinâ vaginal cream indicate their product is for short term management of urogenital symptoms and the monograph clearly states "precautions recommended with oral estrogen administration should also be observed with this route". In one recent study looking at "Serum and tissue hormone levels of vaginally and orally administered estradiol" (Am J Obstet Gynecol 1999;180:1480-3), serum levels were 10 times higher after vaginal vs. oral administration for exactly the same dose while endometrial concentrations were 70 times higher. This suggests that in some cases very little estrogen is required vaginally to produce significant serum levels and there may be preferential absorption into the endometrium. Hence equivalent vaginal doses may sometimes be much lower on a mg per mg basis compared to oral, largely because of bypassing the "first pass" effect. -

Low-Dose Vaginal Estrogen Therapy
where it works locally to improve the quality of the skin by normalizing its acidity and making it thicker and better lu- bricated. The advantage of using local therapy rather than systemic therapy (i.e. hormone tablets or patches, etc.) is that much lower doses of hormone can be used to achieve good effects in the vagina, while minimizing effects on Low-Dose Vaginal other organs such as the breast or uterus. Vaginal estrogen comes in several forms such as vaginal tablet, creams or gel Estrogen Therapy or in a ring pessary. Is local estrogen therapy safe for me? A Guide for Women Vaginal estrogen preparations act locally on the vaginal 1. Why should I use local estrogen? skin, and minimal, if any estrogen is absorbed into the bloodstream. They work in a similar way to hand or face 2. What is intravaginal estrogen therapy? cream. If you have had breast cancer and have persistent 3. Is local estrogen therapy safe for me? troublesome symptoms which aren’t improving with vagi- 4. Which preparation is best for me? nal moisturizers and lubricants, local estrogen treatment 5. If I am already on HRT, do I need local estrogen may be a possibility. Your Urogynecologist will coordinate the use of vaginal estrogen with your Oncologist. Studies so as well? far have not shown an increased risk of cancer recurrence in women using vaginal estrogen who are undergoing treat- ment of breast cancer or those with history of breast cancer. Which preparation is best for me? Your doctor will be able to advise you on this but most women tolerate all forms of topical estrogen. -

Vaginal Sensitivity to Estrogen As Related to Mammary Tumor Incidence in Mice J.J
Vaginal Sensitivity to Estrogen as Related to Mammary Tumor Incidence in Mice J.J. TRENTIN,PH.D.* (From the Department of Anatomy, Yale Unirersity School of Medicine, New Hauen, Connecticut) The demonstration of the importance of ovarian to estrogen bears no relation to the maternal ex- secretion or exogenous estrogen to a high mam trachromosomal factor. mary tumor incidence in mice was followed by Mühlbock(2,3), from the same laboratory, con numerous studies of the estrous cycles of mice of firmed the higher estrogen requirement (5-7 times different strains, in an attempt to correlate high as much) of the high tumor dba strain as compared mammary tumor incidence with some peculiarity to the low tumor C57 and 020 strains for a com of the cycle. In general, no significant and consist parable degree of vaginal stimulation by either in- ent correlation could be demonstrated in this travaginal or subcutaneous administration of regard. Korteweg and co-workers, however, fo estrogen. cused attention on the possibility that, whereas Sliimkin and Andervont (4) also reported on the outward manifestations of the cycle might be rela vaginal estrogen-sensitivity of three strains of mice tively constant, fundamental differences may exist of known mammary tumor incidence—the C57, in the sensitivity of the genital tissues to estrogen, C, and C3H strains. Again the high tumor strain and that such differences may be related to the was more resistant, the C3H strain requiring twice mammary tumor incidence. Van Gulik and Korte as much estrogen as the low tumor C57 and C, weg (5) reported that of one high tumor and two strains for a positive response in 50 per cent of the low tumor inbred strains, compared with regard to mice. -

Is Vaginal Estrogen Safe If I Had Cancer Or a Heart Attack?
Is Vaginal Estrogen Safe If I had Cancer or a Heart Attack? 27th Annual Primary Health Care of Women Conference Samantha Kempner, MD Assistant Professor Department of Obstetrics and Gynecology Michigan Medicine Please consider the environment before printing this PowerPoint Learning Objectives Describe prevalence and impact of vaginal atrophy for menopausal women Review evidence-based approach to management of vaginal menopausal symptoms Discuss safety of vaginal estrogen for patients with cardiovascular disease or breast cancer Clinical Presentation 52yo G3P2012 calls office with 3rd complaint of dysuria in past 2 months. First time urine culture was obtained and showed E.Coli, second time no improvement on empiric antibiotics, third time culture negative. DDx: ?? Atrophy Sex Med. 2013 Dec: 1 (2): 44-53 D D X Please consider the environment before printing this PowerPoint Clin Med Insights Reprod Health. 2014 Jun 8;8:23-30. Sex Med. 2013 Dec: 1 (2): 44-53 Vulvovaginal Atrophy GSM (genitourinary syndrome of menopause) Impacts up to 85% of menopausal women Up to 70% of women do not discuss condition with a healthcare provider Symptoms include: • Vaginal or vulvar dryness • Dyspareunia • Discharge • Worsening Incontinence • Itching • UTIs Rx: Non-Hormonal Hormonal Other Medications Vaginal lubricants (use Local low-dose vaginal Prasterone (Vaginal DHEA) during intercourse) estrogen is the most • FDA approved vaginal Replens, Vagisil effective treatment for suppository Moisturizer, KY moderate to severe GSM: • MOA likely local Liquibeads -
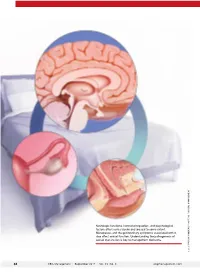
Neurologic Functions, Hormonal Regulation, and Psychological Factors Affect Sexual Desire and Arousal to Some Extent
Neurologic functions, hormonal regulation, and psychological factors affect sexual desire and arousal to some extent. Menopause, and the genitourinary symptoms associated with it, also affect sexual function. Understanding the pathogenesis of sexual dysfunction is key to management decisions. ILLUSTRATION: KIMBERLY MARTENS FOR OBG MANAGEMENT MARTENS KIMBERLY ILLUSTRATION: 34 OBG Management | September 2017 | Vol. 29 No. 9 obgmanagement.com UPDATE FEMALE SEXUAL DYSFUNCTION New and emerging treatment options hold promise for improving outcomes in this undertreated disorder ❯❯ Barbara S. Levy, MD Dr. Levy is Vice President for Health Policy at the American College of Obstetricians and Gynecologists, Washington, DC. The author reports no financial relationships relevant to this article. exual function is a complex, multifac- chronic conditions such as diabetes and S eted process mediated by neurologic back pain.4 functions, hormonal regulation, and psy- Understanding the pathogenesis of chological factors. What could possibly female sexual dysfunction helps to guide go wrong? our approach to its management. Indeed, IN THIS As it turns out, quite a lot. Female sex- increased understanding of its pathology has ARTICLE ual dysfunction is a common, vastly under- helped to usher in new and emerging treat- treated sexual health problem that can have ment options, as well as a personalized, bio- How hormones, wide-reaching effects on a woman’s life. psychosocial approach to its management. experience, These effects may include impaired body In this Update, I discuss the interplay of and behavior affect image, self-confidence, and self-worth. Sex- physiologic and psychological factors that the brain ual dysfunction also can contribute to rela- affect female sexual function as well as the page 36 tionship dissatisfaction and leave one feeling latest options for its management. -

TX-004HR Vaginal Estradiol Has Negligible to Very Low Systemic Absorption of Estradiol
Himmelfarb Health Sciences Library, The George Washington University Health Sciences Research Commons Obstetrics and Gynecology Faculty Publications Obstetrics and Gynecology 12-19-2016 TX-004HR vaginal estradiol has negligible to very low systemic absorption of estradiol. David F Archer Ginger D Constantine James A Simon George Washington University Harvey Kushner Philip Mayer See next page for additional authors Follow this and additional works at: http://hsrc.himmelfarb.gwu.edu/smhs_obgyn_facpubs Part of the Female Urogenital Diseases and Pregnancy Complications Commons, Obstetrics and Gynecology Commons, and the Women's Health Commons APA Citation Archer, D., Constantine, G., Simon, J., Kushner, H., Mayer, P., Bernick, B., Graham, S., Mirkin, S., & REJOICE Study Group. (2016). TX-004HR vaginal estradiol has negligible to very low systemic absorption of estradiol.. Menopause (New York, N.Y.), 24 (5). http://dx.doi.org/10.1097/GME.0000000000000790 This Journal Article is brought to you for free and open access by the Obstetrics and Gynecology at Health Sciences Research Commons. It has been accepted for inclusion in Obstetrics and Gynecology Faculty Publications by an authorized administrator of Health Sciences Research Commons. For more information, please contact [email protected]. Authors David F Archer, Ginger D Constantine, James A Simon, Harvey Kushner, Philip Mayer, Brian Bernick, Shelli Graham, Sebastian Mirkin, and REJOICE Study Group. This journal article is available at Health Sciences Research Commons: http://hsrc.himmelfarb.gwu.edu/smhs_obgyn_facpubs/146 CE: M.S.; MENO-D-16-00223; Total nos of Pages: 7; MENO-D-16-00223 Menopause: The Journal of The North American Menopause Society Vol. 24, No. -

OSPHENA) National Drug Monograph October 2015 VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives
Ospemifene Monograph Ospemifene (OSPHENA) National Drug Monograph October 2015 VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives The purpose of VA PBM Services drug monographs is to provide a comprehensive drug review for making formulary decisions. Updates will be made when new clinical data warrant additional formulary discussion. Documents will be placed in the Archive section when the information is deemed to be no longer current. FDA Approval Information Description/Mechanism of Ospemifene is a selective estrogen receptor modulator (SERM) with agonist Action actions on vaginal tissue, the endometrium and bone. Antagonist actions suggest antitumor effects in experimental breast cancer models. Ospemifene is not interchangeable with other SERMs such as raloxifene or tamoxifen, nor they with it. Indication(s) Under Review in Ospemifene is an estrogen agonist/antagonist indicated for the treatment this document (may include of moderate to severe dyspareunia, a symptom of vulvar and vaginal off label) atrophy, due to menopause. Dosage Form(s) Under Tablet (oral), 60 mg Review REMS REMS X No REMS Postmarketing Requirements See Other Considerations for additional REMS information Pregnancy Rating X Executive Summary Efficacy After 12 weeks ospemifene reduced the symptom score of dyspareunia significantly more than placebo. Thirty-eight percent of postmenopausal women taking ospemifene reported no pain upon intercourse compared to 28% taking placebo. Ospemifene significantly improved the Maturation Index (composed of percent of vaginal parabasal and superficial cells, and vaginal pH) after 12 and 52 weeks of treatment. Vaginal dryness symptom scores improved significantly more with ospemifene than placebo. Use of vaginal lubricant decreased more in women taking ospemifene than placebo. -

Vaginal Dryness
Vaginal Dryness Bothersome symptoms of the vagina and vulva (outer lips of the vagina) increase during and after the menopause transition or may start several years after menopause. The decrease in estrogen with menopause is a major contributor to vaginal dryness, itching, burning, discomfort, and pain during intercourse or other sexual activity. Vaginal atrophy is the medical term that describes these changes. The genitourinary syndrome of menopause includes bothersome vaginal atrophy often combined with urinary symptoms. Vaginal atrophy may significantly affect your quality of life, sexual satisfaction, and relationship with your partner. Unlike hot flashes, which generally improve with time, vaginal symptoms typically worsen with time because of aging and a prolonged lack of estrogen. Menopause and aging can affect the vagina in the following ways: Vaginal tissues become thin, dry, and less elastic, with decreased secretions and lubrication Vaginal infections increase (as the healthy acidic pH of the vagina becomes more alkaline) Discomfort with urination and increased urinary tract infections can occur Fragile, dry, inflamed vaginal tissues may tear and bleed Women with menopause induced by cancer treatments may have additional injury to the vaginal tissues from chemotherapy or pelvic radiation Aromatase inhibitors, taken by many women with breast cancer, result in extremely low estrogen levels, often causing severe symptoms of vaginal dryness and decreased lubrication Vaginal changes often result in pain with sexual activity -

Drugs for the Treatment of Menopausal Symptoms
Review Drugs for the treatment of menopausal symptoms † Susan R Davis & Fiona Jane Monash University, Alfred Hospital, Central Clinical School, Women’s Health Program, 1. Introduction Commercial Road, Prahran, Victoria, Australia 2. Which menopausal symptoms Importance of the field: Over the last decade, the management of the meno- merit treatment? pause has attracted extensive public and professional debate and has become 3. Pharmacotherapy for one of the most controversial areas in clinical practice. menopausal symptoms Areas covered in this review: This review provides an overview of the field, 4. New frontiers primarily from a clinical practice perspective. However, as we have incorpo- 5. Expert opinion rated in this ‘big-picture’ snapshot of the field both conventional and comple- mentary approaches to managing the menopause, it is not an exhaustive review of the literature. What the reader will gain: By reviewing menopausal management from the perspective of practicing clinicians, we hope readers will gain insight into decision making processes appropriate for dealing with symptomatic women. Take home message: Although most women do not require pharmacother- apy for menopausal symptoms, many are severely affected by estrogen defi- ciency at and beyond menopause and, for such women, hormone therapy is important if they are to retain an acceptable quality of life. This article consid- ers the drug treatment of the symptomatic postmenopausal woman and the safety issues related to these medications. Keywords: estrogen, HRT, menopause, progestogen Expert Opin. Pharmacother. (2010) 11(8):1329-1341 For personal use only. 1. Introduction Menopause is not only associated with symptoms that range from bothersome through to extremely distressing, it is also accompanied by hormonal changes that impact adversely on several non-reproductive systems.