Low-Dose Vaginal Estrogen Therapy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Postmenopausal Pharmacotherapy Newsletter
POSTMENOPAUSAL PHARMACOTHERAPY September, 1999 As Canada's baby boomers age, more and more women will face the option of Hormone Replacement Therapy (HRT). The HIGHLIGHTS decision can be a difficult one given the conflicting pros and cons. M This RxFiles examines the role and use of HRT, as well as newer Long term HRT carries several major benefits but also risks SERMS and bisphosphonates in post-menopausal (PM) patients. which should be evaluated on an individual and ongoing basis MContinuous ERT is appropriate for women without a uterus HRT MWomen with a uterus should receive progestagen (at least 12 HRT is indicated for the treatment of PM symptoms such as days per month or continuous low-dose) as part of their HRT vasomotor disturbances and urogenital atrophy, and is considered MLow-dose ERT (CEE 0.3mg) + Ca++ appears to prevent PMO primary therapy for prevention and treatment of postmenopausal MBisphosphinates (e.g. alendronate, etidronate) and raloxifene are osteoporosis (PMO).1 Contraindications are reviewed in Table 2. alternatives to HRT in treating and preventing PMO Although HRT is contraindicated in women with active breast or M"Natural" HRT regimens can be compounded but data is lacking uterine cancer, note that a prior or positive family history of these does not necessarily preclude women from receiving HRT.1 Comparative Safety: Because of differences between products, some side effects may be alleviated by switching from one product Estrogen Replacement Therapy (ERT) 2 to another, particularly from equine to plant sources or from oral to Naturally secreted estrogens include: topical (see Table 3 - Side Effects & Their Management). -

Affect Breast Cancer Risk
HOW HORMONES AFFECT BREAST CANCER RISK Hormones are chemicals made by the body that control how cells and organs work. Estrogen is a female hormone made mainly in the ovaries. It’s important for sexual development and other body functions. From your first monthly period until menopause, estrogen stimulates normal breast cells. A higher lifetime exposure to estrogen may increase breast cancer risk. For example, your risk increases if you start your period at a young age or go through menopause at a later age. Other hormone-related risks are described below. Menopausal hormone therapy Pills Menopausal hormone therapy (MHT) is The U.S. Food and Drug Administration also known as postmenopausal hormone (FDA) recommends women use the lowest therapy and hormone replacement dose that eases symptoms for the shortest therapy. Many women use MHT pills to time needed. relieve hot flashes and other menopausal Any woman currently taking or thinking symptoms. MHT should be used at the Birth control about taking MHT pills should talk with her lowest dose and for the shortest time pills (oral doctor about the risks and benefits. contraceptives) needed to ease menopausal symptoms. Long-term use can increase breast cancer Vaginal creams, suppositories Current or recent use risk and other serious health conditions. and rings of birth control pills There are 2 main types of MHT pills: slightly increases breast Vaginal forms of MHT do not appear to cancer risk. However, estrogen plus progestin and estrogen increase the risk of breast cancer. However, this risk is quite small alone. if you’ve been diagnosed with breast cancer, vaginal estrogen rings and suppositories are because the risk of Estrogen plus progestin MHT breast cancer for most better than vaginal estrogen creams. -

Antiestrogenic Action of Dihydrotestosterone in Mouse Breast
Antiestrogenic action of dihydrotestosterone in mouse breast. Competition with estradiol for binding to the estrogen receptor. R W Casey, J D Wilson J Clin Invest. 1984;74(6):2272-2278. https://doi.org/10.1172/JCI111654. Research Article Feminization in men occurs when the effective ratio of androgen to estrogen is lowered. Since sufficient estrogen is produced in normal men to induce breast enlargement in the absence of adequate amounts of circulating androgens, it has been generally assumed that androgens exert an antiestrogenic action to prevent feminization in normal men. We examined the mechanisms of this effect of androgens in the mouse breast. Administration of estradiol via silastic implants to castrated virgin CBA/J female mice results in a doubling in dry weight and DNA content of the breast. The effect of estradiol can be inhibited by implantation of 17 beta-hydroxy-5 alpha-androstan-3-one (dihydrotestosterone), whereas dihydrotestosterone alone had no effect on breast growth. Estradiol administration also enhances the level of progesterone receptor in mouse breast. Within 4 d of castration, the progesterone receptor virtually disappears and estradiol treatment causes a twofold increase above the level in intact animals. Dihydrotestosterone does not compete for binding to the progesterone receptor, but it does inhibit estrogen-mediated increases of progesterone receptor content of breast tissue cytosol from both control mice and mice with X-linked testicular feminization (tfm)/Y. Since tfm/Y mice lack a functional androgen receptor, we conclude that this antiestrogenic action of androgen is not mediated by the androgen receptor. Dihydrotestosterone competes with estradiol for binding to the cytosolic estrogen receptor of mouse breast, […] Find the latest version: https://jci.me/111654/pdf Antiestrogenic Action of Dihydrotestosterone in Mouse Breast Competition with Estradiol for Binding to the Estrogen Receptor Richard W. -

Estrogen Agents, Oral-Transdermal
GEORGIA MEDICAID FEE-FOR-SERVICE ESTROGEN AGENTS, ORAL - TRANSDERMAL PA SUMMARY Preferred Non-Preferred Oral Estrogens Estradiol generic n/a Menest (esterified estrogens) Premarin (estrogens, conjugated) Oral Estrogen/Progestin Combinations Angeliq (drospirenone/estradiol) Bijuva (estradiol/progesterone) Estradiol/norethindrone and all generics for Activella Norethindrone/ethinyl estradiol and all generics for Femhrt Low Dose 0.5/2.5 (norethindrone/ethinyl Femhrt Low Dose estradiol) Jinteli and all generics for Femhrt 1/5 (norethindrone/ethinyl estradiol) Prefest (estradiol/norgestimate) Premphase (conjugated estrogens/medroxyprogesterone) Prempro (conjugated estrogens/medroxyprogesterone) Topical Estrogens Alora (estradiol transdermal patch) Divigel (estradiol topical gel) Estradiol transdermal patch (generic Climara) Elestrin (estradiol topical gel) Evamist (estradiol topical spray solution) Estradiol transdermal patch (generic Vivelle-Dot) Menostar (estradiol transdermal patch) Minivelle (estradiol transdermal patch) Vivelle-Dot (estradiol transdermal patch) Topical Estrogens/Progestin Combination Climara Pro (estradiol/levonorgestrel transdermal patch) n/a Combipatch (estradiol/norethindrone transdermal patch) Oral Selective Estrogen Receptor Modulator (SERMs) Raloxifene generic Duavee (conjugated estrogens/bazedoxifene) Osphena (ospemifene) LENGTH OF AUTHORIZATION: 1 year PA CRITERIA: Bijuva ❖ Approvable for the treatment of moderate to severe vasomotor symptoms associated with menopause in women with an intact uterus who have experienced inadequate response, allergies, contraindications, drug-drug interactions or intolerable side effects to at least two preferred oral estrogen/progestin combination products. Revised 6/29/2020 Norethindrone/Ethinyl Estradiol and All Generics for Femhrt Low Dose ❖ Prescriber must submit a written letter of medical necessity stating the reasons at least two preferred oral estrogen/progestin combination products, one of which must be brand Femhrt Low Dose, are not appropriate for the member. -

Why the Product Labeling for Low-Dose Vaginal Estrogen Should Be Changed
Menopause: The Journal of The North American Menopause Society Vol. 21, No. 9, pp. 911/916 DOI: 10.1097/gme.0000000000000316 * 2014 by The North American Menopause Society EDITORIAL Why the product labeling for low-dose vaginal estrogen should be changed his commentary summarizes the activities of several of a highly effective local treatment of a common condition clinicians and researchers to encourage modifications with medical risks and adverse effects on quality of life. We Tto the labeling of low-dose vaginal estrogen. Motivated contend that the boxed warning for low-dose vaginal es- by concerns of practicing clinicians that the boxed warning on trogen products is unjustified based on several lines of rea- the labels and package inserts for these products overstate po- soning described below, including the following: (a) the tential risks and thus adversely affect patient care, leaders dramatic differences in blood hormone levels achieved by in the field are spearheading an effort to encourage consider- low-dose vaginal estrogen (eg, Vagifem tablets [estradiol ation of alternative labeling, as discussed below. The members 10 Kg], Estring [releasing estradiol 7.5 Kg/d], or comparable of the Working Group on Women’s Health and Well-Being in low-dose vaginal estrogen cream formulations) versus con- Menopause have affiliations with a number of medical socie- ventional systemic estrogen therapy; (b) absence of ran- ties, including The North American Menopause Society, the domized trial evidence or consistent observational evidence American College of Obstetricians and Gynecologists, the En- linking low-dose vaginal estrogen to cancer, cardiovascular docrine Society, the American Society for Reproductive Med- disease, dementia, or any of the other conditions highlighted icine, the International Society for the Study of Women’s in the boxed warning; and (c) absence of evidence that changes in Sexual Health, and other professional organizations. -

Estrogen Pharmacology. I. the Influence of Estradiol and Estriol on Hepatic Disposal of Sulfobromophthalein (BSP) in Man
Estrogen Pharmacology. I. The Influence of Estradiol and Estriol on Hepatic Disposal of Sulfobromophthalein (BSP) in Man Mark N. Mueller, Attallah Kappas J Clin Invest. 1964;43(10):1905-1914. https://doi.org/10.1172/JCI105064. Research Article Find the latest version: https://jci.me/105064/pdf Journal of Clinical Investigation Vol. 43, No. 10, 1964 Estrogen Pharmacology. I. The Influence of Estradiol and Estriol on Hepatic Disposal of Sulfobromophthalein (BSP) inMan* MARK N. MUELLER t AND ATTALLAH KAPPAS + WITH THE TECHNICAL ASSISTANCE OF EVELYN DAMGAARD (From the Department of Medicine and the Argonne Cancer Research Hospital,§ the University of Chicago, Chicago, Ill.) This report 1 describes the influence of natural biological action of natural estrogens in man, fur- estrogens on liver function, with special reference ther substantiate the role of the liver as a site of to sulfobromophthalein (BSP) excretion, in man. action of these hormones (5), and probably ac- Pharmacological amounts of the hormone estradiol count, in part, for the impairment of BSP dis- consistently induced alterations in BSP disposal posal that characterizes pregnancy (6) and the that were shown, through the techniques of neonatal period (7-10). Wheeler and associates (2, 3), to result from profound depression of the hepatic secretory Methods dye. Chro- transport maximum (Tm) for the Steroid solutions were prepared by dissolving crystal- matographic analysis of plasma BSP components line estradiol and estriol in a solvent vehicle containing revealed increased amounts of BSP conjugates 10% N,NDMA (N,N-dimethylacetamide) 3 in propylene during estrogen as compared with control pe- glycol. Estradiol was soluble in a concentration of 100 riods, implying a hormonal effect on cellular proc- mg per ml; estriol, in a concentration of 20 mg per ml. -

Chronic Unopposed Vaginal Estrogen Therapy
October, 1999. The Rx Files: Q&A Summary S. Downey BSP, L.D. Regier BSP, BA Chronic Unopposed Vaginal Estrogen Therapy The question of whether progestagen opposition is required in a patient on chronic vaginal estrogen is controversial. The literature is not clear on this matter and the SOGC conference on Menopause did not reach a consensus. Endometrial hyperplasia is directly related to the dose and duration of estrogen therapy. The PEPI study showed that 10 per cent of women taking unopposed estrogen (equivalent to 0.625 mg CEE) will develop complex or atypical endometrial hyperplasia within 1 year. With long-term HRT, it is now considered standard practice to add progestagen opposition to oral estrogen therapy in women with an intact uterus. The case is less clear for vaginal estrogen therapy. The makers of Premarinâ vaginal cream indicate their product is for short term management of urogenital symptoms and the monograph clearly states "precautions recommended with oral estrogen administration should also be observed with this route". In one recent study looking at "Serum and tissue hormone levels of vaginally and orally administered estradiol" (Am J Obstet Gynecol 1999;180:1480-3), serum levels were 10 times higher after vaginal vs. oral administration for exactly the same dose while endometrial concentrations were 70 times higher. This suggests that in some cases very little estrogen is required vaginally to produce significant serum levels and there may be preferential absorption into the endometrium. Hence equivalent vaginal doses may sometimes be much lower on a mg per mg basis compared to oral, largely because of bypassing the "first pass" effect. -

Vaginal Sensitivity to Estrogen As Related to Mammary Tumor Incidence in Mice J.J
Vaginal Sensitivity to Estrogen as Related to Mammary Tumor Incidence in Mice J.J. TRENTIN,PH.D.* (From the Department of Anatomy, Yale Unirersity School of Medicine, New Hauen, Connecticut) The demonstration of the importance of ovarian to estrogen bears no relation to the maternal ex- secretion or exogenous estrogen to a high mam trachromosomal factor. mary tumor incidence in mice was followed by Mühlbock(2,3), from the same laboratory, con numerous studies of the estrous cycles of mice of firmed the higher estrogen requirement (5-7 times different strains, in an attempt to correlate high as much) of the high tumor dba strain as compared mammary tumor incidence with some peculiarity to the low tumor C57 and 020 strains for a com of the cycle. In general, no significant and consist parable degree of vaginal stimulation by either in- ent correlation could be demonstrated in this travaginal or subcutaneous administration of regard. Korteweg and co-workers, however, fo estrogen. cused attention on the possibility that, whereas Sliimkin and Andervont (4) also reported on the outward manifestations of the cycle might be rela vaginal estrogen-sensitivity of three strains of mice tively constant, fundamental differences may exist of known mammary tumor incidence—the C57, in the sensitivity of the genital tissues to estrogen, C, and C3H strains. Again the high tumor strain and that such differences may be related to the was more resistant, the C3H strain requiring twice mammary tumor incidence. Van Gulik and Korte as much estrogen as the low tumor C57 and C, weg (5) reported that of one high tumor and two strains for a positive response in 50 per cent of the low tumor inbred strains, compared with regard to mice. -

Is Vaginal Estrogen Safe If I Had Cancer Or a Heart Attack?
Is Vaginal Estrogen Safe If I had Cancer or a Heart Attack? 27th Annual Primary Health Care of Women Conference Samantha Kempner, MD Assistant Professor Department of Obstetrics and Gynecology Michigan Medicine Please consider the environment before printing this PowerPoint Learning Objectives Describe prevalence and impact of vaginal atrophy for menopausal women Review evidence-based approach to management of vaginal menopausal symptoms Discuss safety of vaginal estrogen for patients with cardiovascular disease or breast cancer Clinical Presentation 52yo G3P2012 calls office with 3rd complaint of dysuria in past 2 months. First time urine culture was obtained and showed E.Coli, second time no improvement on empiric antibiotics, third time culture negative. DDx: ?? Atrophy Sex Med. 2013 Dec: 1 (2): 44-53 D D X Please consider the environment before printing this PowerPoint Clin Med Insights Reprod Health. 2014 Jun 8;8:23-30. Sex Med. 2013 Dec: 1 (2): 44-53 Vulvovaginal Atrophy GSM (genitourinary syndrome of menopause) Impacts up to 85% of menopausal women Up to 70% of women do not discuss condition with a healthcare provider Symptoms include: • Vaginal or vulvar dryness • Dyspareunia • Discharge • Worsening Incontinence • Itching • UTIs Rx: Non-Hormonal Hormonal Other Medications Vaginal lubricants (use Local low-dose vaginal Prasterone (Vaginal DHEA) during intercourse) estrogen is the most • FDA approved vaginal Replens, Vagisil effective treatment for suppository Moisturizer, KY moderate to severe GSM: • MOA likely local Liquibeads -
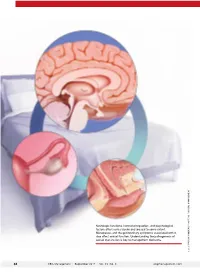
Neurologic Functions, Hormonal Regulation, and Psychological Factors Affect Sexual Desire and Arousal to Some Extent
Neurologic functions, hormonal regulation, and psychological factors affect sexual desire and arousal to some extent. Menopause, and the genitourinary symptoms associated with it, also affect sexual function. Understanding the pathogenesis of sexual dysfunction is key to management decisions. ILLUSTRATION: KIMBERLY MARTENS FOR OBG MANAGEMENT MARTENS KIMBERLY ILLUSTRATION: 34 OBG Management | September 2017 | Vol. 29 No. 9 obgmanagement.com UPDATE FEMALE SEXUAL DYSFUNCTION New and emerging treatment options hold promise for improving outcomes in this undertreated disorder ❯❯ Barbara S. Levy, MD Dr. Levy is Vice President for Health Policy at the American College of Obstetricians and Gynecologists, Washington, DC. The author reports no financial relationships relevant to this article. exual function is a complex, multifac- chronic conditions such as diabetes and S eted process mediated by neurologic back pain.4 functions, hormonal regulation, and psy- Understanding the pathogenesis of chological factors. What could possibly female sexual dysfunction helps to guide go wrong? our approach to its management. Indeed, IN THIS As it turns out, quite a lot. Female sex- increased understanding of its pathology has ARTICLE ual dysfunction is a common, vastly under- helped to usher in new and emerging treat- treated sexual health problem that can have ment options, as well as a personalized, bio- How hormones, wide-reaching effects on a woman’s life. psychosocial approach to its management. experience, These effects may include impaired body In this Update, I discuss the interplay of and behavior affect image, self-confidence, and self-worth. Sex- physiologic and psychological factors that the brain ual dysfunction also can contribute to rela- affect female sexual function as well as the page 36 tionship dissatisfaction and leave one feeling latest options for its management. -

OGEN® Estropipate Tablets, USP
PATIENT INFORMATION (Updated July 2006) OGEN® estropipate tablets, USP Read this PATIENT INFORMATION before you start taking OGEN and read what you get each time you refill OGEN. There may be new information. This information does not take the place of talking to your health care provider about your medical condition or your treatment. WHAT IS THE MOST IMPORTANT INFORMATION I SHOULD KNOW ABOUT OGEN (AN ESTROGEN HORMONE)? • Estrogens increase the chances of getting cancer of the uterus. Report any unusual vaginal bleeding right away while you are taking estrogens. Vaginal bleeding after menopause may be a warning sign of cancer of the uterine (womb). Your health care provider should check any unusual vaginal bleeding to find out the cause. • Do not use estrogens with or without progestins to prevent heart disease, heart attacks or strokes. Using estrogens with or without progestins may increase your chances of getting heart attacks, strokes, breast cancer and blood clots. You and your health care provider should talk regularly about whether you still need treatment with OGEN. What is OGEN? OGEN is a medicine that contains estrogen hormones. What is OGEN used for? OGEN is used during and after menopause to: • reduce moderate or severe hot flashes. Estrogens are hormones made by a woman’s ovaries. The ovaries normally stop making estrogens when a woman is between 45 to 55 years old. This drop in body estrogen levels causes the “change of life” or menopause (the end of monthly menstrual periods). Sometimes, both ovaries are removed during an operation before natural menopause takes place. -

TX-004HR Vaginal Estradiol Has Negligible to Very Low Systemic Absorption of Estradiol
Himmelfarb Health Sciences Library, The George Washington University Health Sciences Research Commons Obstetrics and Gynecology Faculty Publications Obstetrics and Gynecology 12-19-2016 TX-004HR vaginal estradiol has negligible to very low systemic absorption of estradiol. David F Archer Ginger D Constantine James A Simon George Washington University Harvey Kushner Philip Mayer See next page for additional authors Follow this and additional works at: http://hsrc.himmelfarb.gwu.edu/smhs_obgyn_facpubs Part of the Female Urogenital Diseases and Pregnancy Complications Commons, Obstetrics and Gynecology Commons, and the Women's Health Commons APA Citation Archer, D., Constantine, G., Simon, J., Kushner, H., Mayer, P., Bernick, B., Graham, S., Mirkin, S., & REJOICE Study Group. (2016). TX-004HR vaginal estradiol has negligible to very low systemic absorption of estradiol.. Menopause (New York, N.Y.), 24 (5). http://dx.doi.org/10.1097/GME.0000000000000790 This Journal Article is brought to you for free and open access by the Obstetrics and Gynecology at Health Sciences Research Commons. It has been accepted for inclusion in Obstetrics and Gynecology Faculty Publications by an authorized administrator of Health Sciences Research Commons. For more information, please contact [email protected]. Authors David F Archer, Ginger D Constantine, James A Simon, Harvey Kushner, Philip Mayer, Brian Bernick, Shelli Graham, Sebastian Mirkin, and REJOICE Study Group. This journal article is available at Health Sciences Research Commons: http://hsrc.himmelfarb.gwu.edu/smhs_obgyn_facpubs/146 CE: M.S.; MENO-D-16-00223; Total nos of Pages: 7; MENO-D-16-00223 Menopause: The Journal of The North American Menopause Society Vol. 24, No.