TR-546: Sodium Dichromate Dihydrate (CASRN 7789-12-0)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Working with Hazardous Chemicals
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices. In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red “Caution Notes” within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein. -

Safety Data Sheet
SAFETY DATA SHEET Revision date 06-May-2020 Revision Number 1 1. Identification Product identifier Product Name CALCIUM IODATE, REAGENT Other means of identification Product Code(s) C1130 UN/ID no UN1479 Synonyms None Recommended use of the chemical and restrictions on use Recommended use No information available Restrictions on use No information available Details of the supplier of the safety data sheet Supplier Address Spectrum Chemical Mfg. Corp. 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000 Emergency telephone number Emergency Telephone Chemtrec 1-800-424-9300 2. Hazard(s) identification Classification Skin corrosion/irritation Category 2 Serious eye damage/eye irritation Category 2A Specific target organ toxicity (single exposure) Category 3 Oxidizing solids Category 2 Hazards not otherwise classified (HNOC) Not applicable Label elements Danger Hazard statements Causes skin irritation Causes serious eye irritation May cause respiratory irritation May intensify fire; oxidizer Appearance Crystalline powder Physical state Solid Odor Odorless Precautionary Statements - Prevention Wash face, hands and any exposed skin thoroughly after handling Avoid breathing dust/fume/gas/mist/vapors/spray Use only outdoors or in a well-ventilated area Keep away from heat Keep/Store away from clothing/ combustible materials Take any precaution to avoid mixing with combustibles Wear protective gloves/eye protection/face protection Precautionary Statements - Response IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing If eye irritation persists: Get medical advice/attention IF ON SKIN: Wash with plenty of water and soap If skin irritation occurs: Get medical advice/attention Take off contaminated clothing and wash it before reuse IF INHALED: Remove person to fresh air and keep comfortable for breathing Call a POISON CENTER or doctor if you feel unwell In case of fire: Use CO2, dry chemical, or foam to extinguish Precautionary Statements - Storage Store in a well-ventilated place. -

Sodium Chromate 10 Percent Section 1
Conforms to US OSHA Hazard Communication 29CFR1910.1200 SAFETY DATA SHEET Sodium Chromate 10 percent Section 1. Identification 1.1 Product identifier Product name : Sodium Chromate 10 percent Part no. : AR176, AR376 Validation date : 6/24/2020 1.2 Relevant identified uses of the substance or mixture and uses advised against Material uses : Laboratory use Container type: Dispenser Pack AR176 // Sodium Chromate 10 percent // Artisan Grocott's Methenamine Silver Stain Kit // 65 mL and 115 mL AR376 // Sodium Chromate 10 percent // Artisan Grocott's Methenamine Silver Eosin Stain Kit // 65 mL and 115 mL Reference number: SDS056 1.3 Details of the supplier of the safety data sheet Supplier/Manufacturer : Agilent Technologies, Inc. 5301 Stevens Creek Blvd Santa Clara, CA 95051, USA Tel: +1 800 227 9770 Agilent Technologies Singapore (International) Pte Ltd. No. 1 Yishun Avenue 7 Singapore, 768923 Tel. (65) 6276 2622 Agilent Technologies Denmark ApS Produktionsvej 42 2600 Glostrup, Denmark Tel. +45 44 85 95 00 www.Agilent.com e-mail address of person : [email protected] responsible for this SDS 1.4 Emergency telephone number In case of emergency : CHEMTREC®: 1-800-424-9300 Section 2. Hazards identification 2.1 Classification of the substance or mixture OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the substance or mixture Date of issue : 06/24/2020 1/15 Sodium Chromate 10 percent Section 2. Hazards identification H302 ACUTE TOXICITY (oral) - Category 4 H311 -

Coast Guard, DHS Pt. 150, App. I
Coast Guard, DHS Pt. 150, App. I APPENDIX I TO PART 150—EXCEPTIONS Member of reactive group Compatible with TO THE CHART Propylene glycol (20) (a) The binary combinations listed below Oleum (0) ............................... Hexane (31) have been tested as prescribed in Appendix Dichloromethane (36) III and found not to be dangerously reactive. Perchloroethylene (36) These combinations are exceptions to the 1,2-Propylene glycol (20) ...... Diethylenetriamine (7) Compatibility Chart (Figure 1) and may be Polyethylene polyamines (7) Triethylenetetramine (7) stowed in adjacent tanks. Sodium dichromate, 70% (0) Methyl alcohol (20) Member of reactive group Compatible with Sodium hydrosulfide solution Methyl alcohol (20) (5). Acetone (18) .......................... Diethylenetriamine (7) Iso-Propyl alcohol (20) Acetone cyanohydrin (0) ....... Acetic acid (4) Sulfuric acid (2) ..................... Coconut oil (34) Acrylonitrile (15) ..................... Triethanolamine (8) Coconut oil acid (34) Palm oil (34) 1,3-Butylene glycol (20) ......... Morpholine (7) Tallow (34) 1,4-Butylene glycol (20) ......... Ethylamine (7) Sulfuric acid, 98% or less (2) Choice white grease tallow Triethanolamine (8) (34) gamma-Butyrolactone (0) ...... N-Methyl-2-pyrrolidone (9) Caustic potash, 50% or less Isobutyl alcohol (20) (b) The binary combinations listed below (5). Ethyl alcohol (20) have been determined to be dangerously re- Ethylene glycol (20) active, based on either data obtained in the Isopropyl alcohol (20) Methyl alcohol (20) literature or on laboratory testing which has iso-Octyl alcohol (20) been carried out in accordance with proce- Caustic soda, 50% or less (5) Butyl alcohol (20) dures prescribed in Appendix III. These com- tert-Butyl alcohol, Methanol binations are exceptions to the Compat- mixtures ibility Chart (Figure 1) and may not be Decyl alcohol (20) stowed in adjacent tanks. -

Chemical Resistance Chart
CHEMICAL RESISTANCE CHART CHEMICAL RESISTANCE DATA These recommendations are based upon information from material suppliers and careful examination of available published information and are believed to be accurate. However, since the resistance of metals, plastics and elastomers can be affected by concentration, temperature, presence of other chemicals and other factors. This information should be considered as a general guide rather than an unqualified guarantee. Ultimately, the customer must determine the suitability of the pump used in various solutions. All recommendations assume ambient temperatures unless otherwise noted. RATINGS — CHEMICAL EFFECT FOOTNOTES A — No effect—Excellent 1. P.V.C. — Satisfactory to 72 °F B — Minor effect—Good 2. Polypropylene — Satisfactory to 72 °F C — Moderate effect—Fair 3. Polypropylene — Satisfactory to 120 °F D — Severe effect—Not recommended 4. Nitrile — Satisfactory for “O” Rings 5. Polyacetal — Satisfactory to 72 °F 6. Ceramag — Satisfactory to 72 °F The ratings for these materials are based upon the chemical resistance only. Added consideration must be given to pump selections when the chemical is abrasive, viscous in nature, or has a Specific Gravity greater than 1.1. NOTE: The materials shown below in BOLDFACE TYPE are used in the construction of Little Giant chemical pumps. ” 302 Stainless Steel 304 Stainless Steel 316 Stainless Steel 440 Stainless Steel Aluminum TITANIUM C276 NICKEL ALLOY Cast Bronze Brass Cast Iron Carbon Steel 1) PVC (Type (E-3606) Tygon PTFE Polyacetal Nylon ABS Thermoplastic -

Chemical Compatibility Guide
Chemical Compatibility Guide Guide Applicable to the Following: PIG Portable Spill Containment Pool Guide Information This report is offered as a guide and was developed from information which, to the best of New Pig’s knowledge, was reliable and accurate. Due to variables and conditions of application beyond New Pig’s control, none of the data shown in this guide is to be construed as a guarantee, expressed or implied. New Pig assumes no responsibility, obligation, or liability in conjunction with the use or misuse of the information. PIG Spill Containment Pools are constructed from PVC-coated polyester fabric. The chemical resistance guide that follows shows the chemical resistance for the PVC layer only. This guide has been compiled to provide the user with general chemical resistance information. It does not reflect actual product testing. Ratings / Key or Ratings – Chemical Effect 1. Satisfactory to 72°F (22°C) 2. Satisfactory to 120°F (48°C) A = Excellent D = Severe Effect, not recommended for ANY use. B = Good — Minor Effect, slight corrosion or discoloration. N/A = Information not available. C = Fair — Moderate Effect, not recommended for continuous use. Softening, loss of strength, swelling may occur. Due to variables and conditions beyond our control, New Pig cannot guarantee that this product(s) will work to your satisfaction. To ensure effectiveness and your safety, we recommend that you conduct compatibility and absorption testing of your chemicals with this product prior to purchase. For additional questions or information, -

Na2cr04 from Domestic Chromite Concentrates by an Alkali-Fusion Method
R I 1916 T Nor REMOVE FRCl1 r~·ReS~:r~~ n~:nter . ~ E. 315 Montgomery Ave. Spokane, WA 99207 Bureau of Mines Report of Investigations/1988 LI BRARY Na2Cr04 From Domestic Chromite Concentrates by an Alkali-Fusion Method By Gary L. Hundley, R. E. Mussier, R. A. Holmes, and R. S. Olsen UNITED STATES DEPARTMENT OF THE INTERIOR .. Report of Investigations 9167 Na2Cr04 From Domestic Chromite Concentrates by an Alkali-Fusion Method By Gary L. Hundley, R. E. Mussier, R. A. Holmes, and R. S. Olsen UNITED STATES DEPARTMENT OF THE INTERIOR Donald Paul Hodel, Secretary BUREAU OF MINES T S Ary, Director Library of Congress Cataloging in Publication Data: Na2Cr04 from domestic chromite concentrates. (Bureau of Mines report of investigations : 9161) Bibliography: p. 12. Supt. of Does. no.: I 28.23:9167. 1. Chl'omium--Metallurgy. 2. Sodium chromate. 3. Chromite. 4. Sodium hydroxide. 5. Fused salts. 6. Leaching. 1. Hundley, Gary L. II. Title: Alkali-fusion method. III. Series: Report of investigations (United States. Bureau of Mines) ; 9167. TN23.U43 [TN799.C5] 62 s [669'.734] 88-600002 gas CONTENTS Abstract ......•...•..••......•..•....................•..... 1 Introduction ••••••••••••••••••••••••••••• · .............. 2 Raw materials ..••••.••••.•.••.•.•...•••.. 4 Countercurrent leaching •••••••••••••••••• .................... 5 Equipment and procedures ••••••••••••••• 5 Results and discussion ••••••••••••••••••• 6 Solution purification •••••••••••••••••••• 7 Crystallization •••••••••••••••••••••••••• ·. .. .. .. ............. 9 Equipment and procedures -

Solubility Product Constant (Ksp) for a Salt of Limited Solubility
CHM130 Solubility Product Experiment Experiment: Solubility Product Constant (Ksp) for a Salt of Limited Solubility Introduction: The equilibrium process in this experiment is a saturated aqueous solution of calcium iodate, Ca(IO3)2. The relevant solubility equation and solubility product expression, are both shown below. - 2+ 2+ 2 Ca(IO3)2(s) <-===== > Ca (aq) + 2IO3 (aq) Ksp = [Ca ] [IO3 ] For a saturated solution of calcium iodate, if you can determine either the molar concentration of calcium ion, or the molar concentration iodate ion, the solubility product constant can be found using the reverse of the process shown above. There was found the silver ion concentration, in a saturated aqueous solution, from a known value for Ksp. In other words, if the calcium ion concentration in today's experiment was found to be 0.1 M, you could immediately say the concentration of iodate ion must be half that value, or 0.05 M, according to the stoichiometry of the solubility equation given above. The solubility product constant could then be found with simple arithmetic. In this experiment, the iodate ion concentration of a saturated calcium iodate solution will be found via a redox titration with sodium thiosulfate, Na2S2O3. The concentration of iodate ion (IO3-) will be determined by titration with a standardized sodium thiosulfate (Na2S2O3) solution in the presence of potassium iodide (KI). Starch will be used as an indicator, and a sharp blue-to-clear transition will mark the equivalence point. The relevant reaction equations are summarized as follows. IO3 (aq) + 5I (aq) + 6H3O (aq) --------> 3I2(aq) + 9H2O(l) This step, which occurs after adding both solid KI, and aqueous acid, to aliquots of saturated iodate solutions, has the net effect of converting iodate ions to aqueous iodine. -
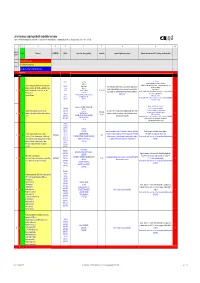
List of Substances Subject to Prohibited / Undesirable / Declaration
List of substances subject to prohibited / undesirable / declaration Appendix 1 to the Guidelines to Design and Purchasing Prohibition of Harmful Substances : ebmpapstMulfingen: 90-001 ebmpapstSt.Georgen: WN 3-12.2 ebmpapstLandshut: 60118.00040 (Ver. 5.0-31.07.2008) 1 2 3 4 5 6 7 8 9 10 Consecu N - new Substance EU-INDEX-No.: CAS-No. Source (law, decree, guideline). Hazard/risk example of application/occurrence Remarks and comments (of 0.1% deviating consideration limit) tive No. V = prohibited (verboten) U = undesirable (unerwünscht) D = subject to declaration (deklarationspflichtig) V = prohibited RoHS from 100 ppm 7439-92-1 TRGS 905, Pb-stabilisers and pigments are subject to declaration. GefStoffV, Prohibited: Anhydrite neutral lead carbonate, lead hydrogen carbonate and lead Lead and compounds (red lead oxide, lead sulphate, lead (7446-14-2 ChemVerbotsV, cable coating, heat transfer medium for accumulators, hybrid integrated sulphate as dye pigment hydrogen carbonate, lead carbonate, lead phtalates, lead 1319-46-6 BatterieV, circuits, stabilisers in plastics, vessels and pipes for aggressive liquids, < 0.4 % in batteries 1 V N acetates, lead phosphates, lead stereates , etc) lead 598-63-0 2002/95/EG (RoHS), K3, R 1, R 3 V E F according to RoHS: Lead as alloying additive in aluminum (up to 0.4 mass%) in steel (up to 67/458/EEC pigment production, lead alloys, anti-corrosion agents (fuel additives), chromate: refer to 0.35 mass%), in copper (up to 4%), 301-04-2 76/769/EEC (89/667/EEC, 97/10/EC, 97/56/EC) soldering agent chromium (VI) -

Consideration of Mandatory Fortification with Iodine for Australia and New Zealand Food Technology Report
CONSIDERATION OF MANDATORY FORTIFICATION WITH IODINE FOR AUSTRALIA AND NEW ZEALAND FOOD TECHNOLOGY REPORT December 2007 1 Introduction Food Standards Australia New Zealand is considering mandatory fortification of the food supply in Australia and New Zealand with iodine. Generally, the addition of iodine to foods is technologically feasible. However, in some instances the addition of iodine can lead to quality changes in food products such as appearance, taste, odour, texture and shelf life. These changes will depend on the chemical form of iodine used as a fortificant, the chemistry of the food that is being fortified, the food processes involved in manufacture and possible processing interactions that could occur during distribution and storage. Many foods have been fortified with iodine and the potassium salts of iodine compounds have been used as the preferred form. 2 Forms of Iodine Iodine is normally introduced, or supplemented, as the iodide or iodate of potassium, calcium or sodium. The following table lists different chemical forms of iodine along with their important physical properties. Table 1: Physical Properties of Iodine and its Compounds Name Chemical Formula % Iodine Solubility in water (g/L) 0°C 20°C 30°C 40°C 60°C Iodine I2 100 - - 0.3 0.4 0.6 Calcium iodide CaI2 86.5 646 676 690 708 740 Calcium iodate Ca(IO3)2.6H2O 65.0 - 1.0 4.2 6.1 13.6 Potassium iodide KI 76.5 1280 1440 1520 1600 1760 Potassium iodate KIO3 59.5 47.3 81.3 117 128 185 Sodium iodide NaI.2H20 85.0 1590 1790 1900 2050 2570 Sodium iodate NaIO3 64.0 - 25.0 90.0 150 210 Adapted from Mannar and Dunn (1995) 2.1 Potassium Iodide Potassium iodide (KI) is highly soluble in water. -

Calcium Chloride
Iodine Livestock 1 2 Identification of Petitioned Substance 3 4 Chemical Names: 7553-56-2 (Iodine) 5 Iodine 11096-42-7 (Nonylphenoxypolyethoxyethanol– 6 iodine complex) 7 Other Name: 8 Iodophor Other Codes: 9 231-442-4 (EINECS, Iodine) 10 Trade Names: CAS Numbers: 11 FS-102 Sanitizer & Udderwash 12 Udder-San Sanitizer and Udderwash 13 14 Summary of Petitioned Use 15 The National Organic Program (NOP) final rule currently allows the use of iodine in organic livestock 16 production under 7 CFR §205.603(a)(14) as a disinfectant, sanitizer and medical treatment, as well as 7 CFR 17 §205.603(b)(3) for use as a topical treatment (i.e., teat cleanser for milk producing animals). In this report, 18 updated and targeted technical information is compiled to augment the 1994 Technical Advisory Panel 19 (TAP) Report on iodine in support of the National Organic Standard’s Board’s sunset review of iodine teat 20 dips in organic livestock production. 21 Characterization of Petitioned Substance 22 23 Composition of the Substance: 24 A variety of substances containing iodine are used for antisepsis and disinfection. The observed activity of 25 these commercial disinfectants is based on the antimicrobial properties of molecular iodine (I2), which 26 consists of two covalently bonded atoms of elemental iodine (I). For industrial uses, I2 is commonly mixed 27 with surface-active agents (surfactants) to enhance the water solubility of I2 and also to sequester the 28 available I2 for extended release in disinfectant products. Generally referred to as iodophors, these 29 “complexes” consist of up to 20% I2 by weight in loose combination with nonionic surfactants such as 30 nonylphenol polyethylene glycol ether (Lauterbach & Uber, 2011). -

Massachusetts Chemical Fact Sheet
Massachusetts Chemical Fact Sheet Hexavalent Chromium Table 1: HEXAVALENT CHROMIUM COMPOUNDS: Compounds SELECTED EXAMPLES* Compound Chemical Formula CAS # This fact sheet is part of a series of chemical fact sheets Ammonium chromate (NH ) Cr0 7788-98-9 developed by TURI to help Massachusetts companies, 4 2 4 community organizations and residents understand the Ammonium dichromate (NH4)2Cr2O7 7789-09-5 chemical’s use and health and environmental effects, as Barium chromate BaCrO4 10294-40-3 well as the availability of safer alternatives. tert-Butyl Chromate [(CH3)3CO]2CrO2 1189-85-1 Hexavalent chromium compounds are a toxic form of Calcium chromate CaCrO4 13765-19-0 chromium and are used in a variety of industrial processes Chromic acid H2CrO4 7738-94-5 and products. Chromium VI chloride CrCl6 14986-48-2 Hexavalent chromium compounds are human carcinogens, Chromic trioxide CrO3 1333-82-0 mutagens and developmental toxicants and are acutely Hexavalent chromium ion Cr6+ 18540-29-9 toxic. Non-hexavalent chromium compounds do not pose Lead chromate PbCrO4 7758-97-6 the same level of concern with regard to either chronic or Lead chromate oxide PbCrO4-PbO 8454-12-1 acute toxicity. Potassium chlorochromate KCrO3Cl 16037-50-6 Until 2011, all chromium compounds were treated as Potassium chromate K2CrO4 7789-00-6 a single category under TURA. Beginning with Potassium dichromate K Cr O 7778-50-9 reporting year 2012, hexavalent chromium 2 2 7 compounds are reportable under TURA as a Silver chromate Ag2CrO4 7784-01-2 separate category and are designated as a Higher Sodium chromate Na2CrO4 7775-11-3 Hazard Substance, which lowers the reporting Sodium dichromate 7789-12-0 threshold to 1,000 lb/year.