Bond Dissociation Energies in Simple Molecules
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

(VI) and Chromium (V) Oxide Fluorides
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 1976 The chemistry of chromium (VI) and chromium (V) oxide fluorides Patrick Jay Green Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Chemistry Commons Let us know how access to this document benefits ou.y Recommended Citation Green, Patrick Jay, "The chemistry of chromium (VI) and chromium (V) oxide fluorides" (1976). Dissertations and Theses. Paper 4039. https://doi.org/10.15760/etd.5923 This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. All ABSTRACT OF THE TllESIS OF Patrick Jay Green for the Master of Science in Chemistry presented April 16, 1976. Title: Chemistry of Chromium(VI) and Chromium(V) Oxide Fluorides. APPROVEO BY MEMBERS OF THE THESIS CO'"o\l TIEE: y . • Ii . ' I : • • • • • New preparative routes to chromyl fluoride were sought. It was found that chlorine ironofluoride reacts with chromium trioxide and chromyl chlo ride to produce chromyl fluoride. Attempts were ~ade to define a mechan ism for the reaction of ClF and Cr0 in light of by-products observed 3 and previous investigations. Carbonyl fluoride and chromium trioxide react to fom chro·yl fluoride and carbo:i dioxide. A mechanism was also proposed for this react10n. Chromium trioxide 11itl\ l~F6 or WF5 reacts to produce chromyl fluoride and the respective oxide tetrafluoride. 2 Sulfur hexafluoride did not react with Cr03. -

China and Weapons of Mass Destruction: Implications for the United States
China and Weapons of Mass Destruction: Implications for the United States China and Weapons of Mass Destruction: Implications for the United States 5 November 1999 This conference was sponsored by the National Intelligence Council and Federal Research Division. The views expressed in this report are those of individuals and do not represent official US intelligence or policy positions. The NIC routinely sponsors such unclassified conferences with outside experts to gain knowledge and insight to sharpen the level of debate on critical issues. Introduction | Schedule | Papers | Appendix I | Appendix II | Appendix III | Appendix IV Introduction This conference document includes papers produced by distinguished experts on China's weapons-of-mass-destruction (WMD) programs. The seven papers were complemented by commentaries and general discussions among the 40 specialists at the proceedings. The main topics of discussion included: ● The development of China's nuclear forces. ● China's development of chemical and biological weapons. ● China's involvement in the proliferation of WMD. ● China's development of missile delivery systems. ● The implications of these developments for the United States. Interest in China's WMD stems in part from its international agreements and obligations. China is a party to the International Atomic Energy Agency (IAEA), the Treaty on the Non-Proliferation of Nuclear Weapons (NPT), the Zangger Committee, and the Chemical Weapons Convention (CWC) and has signed but not ratified the Comprehensive Nuclear Test Ban Treaty (CTBT). China is not a member of the Australia Group, the Wassenaar Arrangement, the Nuclear Suppliers Group, or the Missile Technology Control Regime (MTCR), although it has agreed to abide by the latter (which is not an international agreement and lacks legal authority). -

I. MATRIX ISOLATION of 1,1-DIAZENES II. DISTANCE, TEMPERATURE, and DYNAMIC SOLVENT EFFECTS on ELECTRON TRANSFER REACTIONS Thesis
I. MATRIX ISOLATION OF 1,1-DIAZENES II. DISTANCE, TEMPERATURE, AND DYNAMIC SOLVENT EFFECTS ON ELECTRON TRANSFER REACTIONS Thesis by James Edward Hanson In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy California Institute of Technology Pasadena, California 1990 (Submitted September 8, 1989) 11 © 1990 James Edward Hanson All rights Reserved ill Acknowledgements I would like to thank my advisor, Peter Dervan , for his enthusiasm and support during my studies at Caltech. I would also like to thank Professor John Hopfield for his availability when I needed assistance with the theoretical complexities of the electron transfer work. I am deeply indebted to Lutfur "Zeta" Khundkar and Joe Perry for their expert assistance and collaboration in making measurements and understanding the implications of the results. Al Sylwester was extremely helpful in my first year as I began to work on 1,1-diazenes, and Alvin Joran and Burt Leland made my transition to the electron transfer project relatively simple. I should also thank those in the Dervan group who helped me with synthetic problems, especially John Griffin and Warren Wade. I appreciated discussions with some of the electron transfer experts at Caltech: Professor Rudy Marcus, Dave Beratan, Jose Onuchic, Dave Malerba, and Tad Fox. There were many times when the men in the chemistry shops provided invaluable assistance--you guys are the best! There are many who made my stay at Caltech enjoyable--I'll remember skiing with John and Linda Griffin and Heinz Moser, basketball (and marathons!) with Dave Kaisaki, tennis with Erich Uffelmann, and late night conversations with Kevin Luebke. -

Safety Data Sheet
SAFETY DATA SHEET Preparation Date: 05/19/2015 Revision Date: 05/08/2017 Revision Number: G2 1. IDENTIFICATION Product identifier Product code: B1100 Product Name: BISMUTH METAL, GRANULAR, REAGENT Other means of identification Synonyms: Bismuth-209 Bismuth, elemental CAS #: 7440-69-9 RTECS # EB2600000 CI#: Not available Recommended use of the chemical and restrictions on use Recommended use: In safety devices in fire detection and extinguishing systems; Catalyst for making acrylic fibers; In production of malleable irons; carrier for radioactive uranium fuel in atomic reactor; In printing industry; alloying agent; chemical intermediate for pharmaceuticals and other chemicals; In cosmetics. Uses advised against No information available Supplier: Spectrum Chemical Mfg. Corp 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000. Order Online At: https://www.spectrumchemical.com Emergency telephone number Chemtrec 1-800-424-9300 Contact Person: Martin LaBenz (West Coast) Contact Person: Ibad Tirmiz (East Coast) 2. HAZARDS IDENTIFICATION Classification This chemical is not considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Not a dangerous substance or mixture according to the Globally Harmonized System (GHS) Label elements Not classified Hazards not otherwise classified (HNOC) Not Applicable Other hazards May be harmful if swallowed Product code: B1100 Product name: BISMUTH METAL, 1 / 11 GRANULAR, REAGENT 3. COMPOSITION/INFORMATION ON INGREDIENTS Components CAS-No. Weight % Bismuth Metal 7440-69-9 100 4. FIRST AID MEASURES First aid measures General Advice: National Capital Poison Center in the United States can provide assistance if you have a poison emergency and need to talk to a poison specialist. -

United States Patent Office Patented Dec
3,629,301 United States Patent Office Patented Dec. 21, 1971 3,629,301 3,3-DIFLUORO-2-SUBSTITUTED STEROIDS AND THER PREPARATION William C. Ripka, Wilmington, Del, assignor to E. I. du Pont de Nemours and Company, Wilmington, Del. 5 where R is hydrogen, methyl, or ethynyl, and R2 is hydro No Drawing. Filed Oct. 24, 1969, Ser. No. 869,352 gen or an alkanoyl having no more than 8 carbon atoms, Int, C. C07c 169/20 Such as formyl, acetyl, propionyl, butyryl, valleryl and U.S. C. 260-397.3 10 Claims caproyl. These compounds are prepared from 3-fluoro-A2-steroids 10 by a reaction with nitrosyl fluoride and subsequent conver ABSTRACT OF THE DISCLOSURE sion of the 3,3-difluoro-2-nitrimino steroid products to the New steroids of the formula corresponding 3,3-difluoro-2-keto steroids with alumina CE containing Water, as shown in the reaction scheme below X involving the steroid A-ring: R NO R R ? NOF N= A. AlO3.H2O Os R - A. l -3 F2 Fs! N Z-F 20 The 2-keto group can be converted to the 2-hydroxy F= group by conventional techniques, including, for example, wherein Z is oxygen or reduction with sodium borohydrides. DETALED DESCRIPTION OF THE INVENTION The first step of the reaction sequence, the reaction of a .O 3-fluoro-Asteroid with nitrosyl fluoride is carried out in R is hydrogen or methyl; and X is oxygen or an inert Solvent such as methylene chloride, chloroform, OR2 carbon tetrachloride, fluorodichloromethane, and ethylene 30 dichloride. -
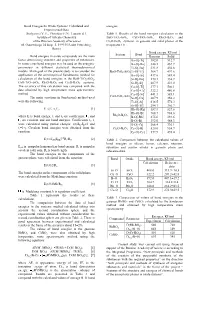
Bond Energies in Oxide Systems: Calculated and Experimental Data
Bond Energies In Oxide Systems: Calculated and energies. Experimental Data Stolyarova V. L., Plotnikov E.N., Lopatin S.I. Table 1. Results of the bond energies calculation in the Institute of Silicate Chemistry BaO-TiO 2-SiO 2, CaO-TiO 2-SiO 2, Rb 2O-B2O3 and of the Russian Academy of Sciences Cs 2O-B2O3 systems. in gaseous and solid phases at the ul. Odoevskogo 24 korp. 2, 199155 Sankt Petersburg, temperature 0 ¥ . Russia Bond energy, KJ/mol System Bond Bond energies in oxide compounds are the main Gaseous Solid factor determining structure and properties of substances. Ba-O[-Ti] 382.0 512.7 In some cases bond energies may be used as the energetic Ba-O[-Ba] 348.5 467.7 parameters in different statistical thermodynamical Ti-O[-Ba] 331.8 450.6 models. Main goal of the present study is to consider the BaO-TiO 2-SiO 2 Ti-O[-Ti] 364.7 497.6 application of the semiempirical Sanderson's method for Ba-O[-Si] 437.6 585.4 calculation of the bond energies in the BaO-TiO 2-SiO 2, Si-O[-Ba] 270.3 334.7 CaO-TiO 2-SiO 2, Rb 2O-B2O3 and Cs 2O-B2O3 systems. Si-O[-Si] 407.9 421.0 The accuracy of this calculation was compared with the Ca-O[-Ti] 377.1 500.1 data obtained by high temperature mass spectrometric Ca-O[-Ca] 322.2 486.8 method. Ca-O[-Si] 441.1 585.1 CaO-TiO -SiO The main equations in Sanderson's method used 2 2 Si-O[-Ca] 287.9 358.2 were the following. -

Ger'g .Tyson Jr
July 14, 1964 ' G. N. TYSON, JR 3,140,582 ROCKET PROPULSION METHOD USING BORON AND NITROGEN COMPOUNDS Filed April 14, 1959 Ger'g .Tyson Jr. INVENTOR. I", u .12) ATTORNEYS 3,l4,582 Patented July 14,, 1964 2 The nitrogen containing compound and the boron con 3,140,582 taining compound are reacted in such proportions that all ROCKET PROPULSION METHOD USING BORON of the nitrogen and all of the boron react to produce AND NOGEN COMPOUNDS boron nitride, the carbon is released as elemental carbon George N. Tyson, Jr., Claremont, Calif., assignor to Olin Mathieson Chemical Corporation, a corporation of and large volumes of hydrogen gas are produced. Virginia Nitrogen containing compounds which can be employed Filed Apr. 14, 1959, Ser. No. 806,396 as reactants include the saturated hydronitrogens such 20 Claims. (Cl. 60-354) as ammonia, hydrazine, triazane, tetrazane; the unsaturated hydronitrogens such as diimide, triazene, tetrazene, iso This invention relates to a method for producing large 10 tetrazene, ammonium azide, hydrazine azide, and hydra volumes of hot gases in a short period of time, which zoic acid; alkyl hydrazines such as methyl hydrazine, un large volumes of hot gases are useful for many purposes symmetrical dimethyl hydrazine, ethyl hydrazine, unsym including imparting thrust to jet propelled devices such metrical diethyl hydrazine; alkylamines including mixed al as rockets. kylamines such as methylamine, dimethylamine, trimeth Jet propelled devices are essentially of two types: those 15 ylamine, ethylamine, diethylamine, triethylarnine, methyl which depend upon an external source for a portion of ethyl amine, n-propylamine, isopropylamine, di-n-propyl the propellant, and those in which the propellant is en amine, tri-n-propylamine, methyl propyl amine, ethyl prop tirely contained within the device. -

Molecular Orbital Theory to Predict Bond Order • to Apply Molecular Orbital Theory to the Diatomic Homonuclear Molecule from the Elements in the Second Period
Skills to Develop • To use molecular orbital theory to predict bond order • To apply Molecular Orbital Theory to the diatomic homonuclear molecule from the elements in the second period. None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. These approaches also cannot describe the nature of resonance. Such limitations led to the development of a new approach to bonding in which electrons are not viewed as being localized between the nuclei of bonded atoms but are instead delocalized throughout the entire molecule. Just as with the valence bond theory, the approach we are about to discuss is based on a quantum mechanical model. Previously, we described the electrons in isolated atoms as having certain spatial distributions, called orbitals, each with a particular orbital energy. Just as the positions and energies of electrons in atoms can be described in terms of atomic orbitals (AOs), the positions and energies of electrons in molecules can be described in terms of molecular orbitals (MOs) A particular spatial distribution of electrons in a molecule that is associated with a particular orbital energy.—a spatial distribution of electrons in a molecule that is associated with a particular orbital energy. As the name suggests, molecular orbitals are not localized on a single atom but extend over the entire molecule. Consequently, the molecular orbital approach, called molecular orbital theory is a delocalized approach to bonding. Although the molecular orbital theory is computationally demanding, the principles on which it is based are similar to those we used to determine electron configurations for atoms. -

Chemical Chemical Hazard and Compatibility Information
Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 degrees F) to avoid rupture of carboys and glass containers.. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino-ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers (strong), P(OCN)3, Perchloric acid, Permanganates, Peroxides, Phenols, Phosphorus isocyanate, Phosphorus trichloride, Potassium hydroxide, Potassium permanganate, Potassium-tert-butoxide, Sodium hydroxide, Sodium peroxide, Sulfuric acid, n-Xylene. Acetone HAZARDS & STORAGE: Store in a cool, dry, well ventilated place. INCOMPATIBILITIES: Acids, Bromine trifluoride, Bromine, Bromoform, Carbon, Chloroform, Chromium oxide, Chromium trioxide, Chromyl chloride, Dioxygen difluoride, Fluorine oxide, Hydrogen peroxide, 2-Methyl-1,2-butadiene, NaOBr, Nitric acid, Nitrosyl chloride, Nitrosyl perchlorate, Nitryl perchlorate, NOCl, Oxidizing materials, Permonosulfuric acid, Peroxomonosulfuric acid, Potassium-tert-butoxide, Sulfur dichloride, Sulfuric acid, thio-Diglycol, Thiotrithiazyl perchlorate, Trichloromelamine, 2,4,6-Trichloro-1,3,5-triazine -

"Fluorine Compounds, Organic," In: Ullmann's Encyclopedia Of
Article No : a11_349 Fluorine Compounds, Organic GU¨ NTER SIEGEMUND, Hoechst Aktiengesellschaft, Frankfurt, Federal Republic of Germany WERNER SCHWERTFEGER, Hoechst Aktiengesellschaft, Frankfurt, Federal Republic of Germany ANDREW FEIRING, E. I. DuPont de Nemours & Co., Wilmington, Delaware, United States BRUCE SMART, E. I. DuPont de Nemours & Co., Wilmington, Delaware, United States FRED BEHR, Minnesota Mining and Manufacturing Company, St. Paul, Minnesota, United States HERWARD VOGEL, Minnesota Mining and Manufacturing Company, St. Paul, Minnesota, United States BLAINE MCKUSICK, E. I. DuPont de Nemours & Co., Wilmington, Delaware, United States 1. Introduction....................... 444 8. Fluorinated Carboxylic Acids and 2. Production Processes ................ 445 Fluorinated Alkanesulfonic Acids ...... 470 2.1. Substitution of Hydrogen............. 445 8.1. Fluorinated Carboxylic Acids ......... 470 2.2. Halogen – Fluorine Exchange ......... 446 8.1.1. Fluorinated Acetic Acids .............. 470 2.3. Synthesis from Fluorinated Synthons ... 447 8.1.2. Long-Chain Perfluorocarboxylic Acids .... 470 2.4. Addition of Hydrogen Fluoride to 8.1.3. Fluorinated Dicarboxylic Acids ......... 472 Unsaturated Bonds ................. 447 8.1.4. Tetrafluoroethylene – Perfluorovinyl Ether 2.5. Miscellaneous Methods .............. 447 Copolymers with Carboxylic Acid Groups . 472 2.6. Purification and Analysis ............. 447 8.2. Fluorinated Alkanesulfonic Acids ...... 472 3. Fluorinated Alkanes................. 448 8.2.1. Perfluoroalkanesulfonic Acids -

Calculating Lattice Energies Using the Born-Haber Cycle an Extension Activity for AP Chemistry Students
Calculating Lattice Energies Using the Born-Haber Cycle An Extension Activity for AP Chemistry Students A particular set of equations known as a Born-Haber cycle demonstrates how chemists are able to use the first law of thermodynamics—that the energy of the universe is conserved in any chemical or physical change—to find an unknown energy value that is difficult or impossible to measure experimentally. Some energy quantities, such as the lattice energy of a mineral or the electron affinity of an atom, can be difficult to measure in the lab. Examining a Born-Haber cycle we see that there is more than one path to the formation of a substance in a particular state, and that if we use consistent definitions, an energy value that we seek can be calculated from energy values that we already know. The following exercise will help us see the way these energy values relate to one another, give us practice with their definitions and symbols, and deepen our insight to their meaning when we see them in other types of problems. Each physical or chemical change represented has: • an equation that represents a clearly defined physical or chemical change; • a definition of the particular type of energy change; • a symbol or abbreviation for the energy change equal to a value for the change in enthalpy (ΔH), the energy that is released or absorbed during the change, expressed in kJ/mol; and • a name by which that change in enthalpy is commonly known. To set up the equations in a Born-Haber cycle, cut out the cards for names, equations, defini- tions, and symbols with energy values.