Hymap and ASTER Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download PDF About Minerals Sorted by Mineral Name
MINERALS SORTED BY NAME Here is an alphabetical list of minerals discussed on this site. More information on and photographs of these minerals in Kentucky is available in the book “Rocks and Minerals of Kentucky” (Anderson, 1994). APATITE Crystal system: hexagonal. Fracture: conchoidal. Color: red, brown, white. Hardness: 5.0. Luster: opaque or semitransparent. Specific gravity: 3.1. Apatite, also called cellophane, occurs in peridotites in eastern and western Kentucky. A microcrystalline variety of collophane found in northern Woodford County is dark reddish brown, porous, and occurs in phosphatic beds, lenses, and nodules in the Tanglewood Member of the Lexington Limestone. Some fossils in the Tanglewood Member are coated with phosphate. Beds are generally very thin, but occasionally several feet thick. The Woodford County phosphate beds were mined during the early 1900s near Wallace, Ky. BARITE Crystal system: orthorhombic. Cleavage: often in groups of platy or tabular crystals. Color: usually white, but may be light shades of blue, brown, yellow, or red. Hardness: 3.0 to 3.5. Streak: white. Luster: vitreous to pearly. Specific gravity: 4.5. Tenacity: brittle. Uses: in heavy muds in oil-well drilling, to increase brilliance in the glass-making industry, as filler for paper, cosmetics, textiles, linoleum, rubber goods, paints. Barite generally occurs in a white massive variety (often appearing earthy when weathered), although some clear to bluish, bladed barite crystals have been observed in several vein deposits in central Kentucky, and commonly occurs as a solid solution series with celestite where barium and strontium can substitute for each other. Various nodular zones have been observed in Silurian–Devonian rocks in east-central Kentucky. -

Geochemical Alteration of Pyrochlore Group Minerals: Pyrochlore Subgroup
American Mineralogist, Volume 80, pages 732-743, 1995 Geochemical alteration of pyrochlore group minerals: Pyrochlore subgroup GREGORY R. LUMPKIN Advanced Materials Program, Australian Nuclear Science and Technology Organization, Menai 2234, New South Wales, Australia RODNEY C. EWING Department of Earth and Planetary Sciences, University of New Mexico, Albuquerque, New Mexico 87131, U.S.A. ABSTRACT Primary alteration of uranpyrochlore from granitic pegmatites is characterized by the substitutions ADYD-+ ACaYO, ANaYF -+ ACaYO, and ANaYOI-I --+ ACaYO. Alteration oc- curred at ""450-650 °C and 2-4 kbar with fluid-phase compositions characterized by relatively low aNa+,high aeaH, and high pH. In contrast, primary alteration of pyrochlore from nepheline syenites and carbonatites follows a different tre:nd represented by the sub- stitutions ANaYF -+ ADYD and ACaYO -+ ADYD. In carbonatites, primary alteration of pyrochlore probably took place during and after replacement of diopside + forsterite + calcite by tremolite + dolomite :t ankerite at ""300-550 °C and 0-2 kbar under conditions of relatively low aHF, low aNa+,low aeaH, low pH, and elevated activities of Fe and Sr. Microscopic observations suggest that some altered pyrochlor1es are transitional between primary and secondary alteration. Alteration paths for these specimens scatter around the trend ANaYF -+ ADYD. Alteration probably occurred at 200-350 °C in the presence of a fluid phase similar in composition to the fluid present during primary alteration but with elevated activities of Ba and REEs. Mineral reactions in the system Na-Ca-Fe-Nb-O-H indicate that replacement of pyrochlore by fersmite and columbite occurred at similar conditions with fluid conpositions having relatively low aNa+,moderate aeaH, and mod- erate to high aFeH.Secondary alteration « 150 °C) is charactlerized by the substitutions ANaYF -+ ADYD,ACaYO -+ ADYD,and ACaXO -+ ADXDtogether with moderate to extreme hydration (10-15 wt% H20 or 2-3 molecules per formula unit). -

Washington State Minerals Checklist
Division of Geology and Earth Resources MS 47007; Olympia, WA 98504-7007 Washington State 360-902-1450; 360-902-1785 fax E-mail: [email protected] Website: http://www.dnr.wa.gov/geology Minerals Checklist Note: Mineral names in parentheses are the preferred species names. Compiled by Raymond Lasmanis o Acanthite o Arsenopalladinite o Bustamite o Clinohumite o Enstatite o Harmotome o Actinolite o Arsenopyrite o Bytownite o Clinoptilolite o Epidesmine (Stilbite) o Hastingsite o Adularia o Arsenosulvanite (Plagioclase) o Clinozoisite o Epidote o Hausmannite (Orthoclase) o Arsenpolybasite o Cairngorm (Quartz) o Cobaltite o Epistilbite o Hedenbergite o Aegirine o Astrophyllite o Calamine o Cochromite o Epsomite o Hedleyite o Aenigmatite o Atacamite (Hemimorphite) o Coffinite o Erionite o Hematite o Aeschynite o Atokite o Calaverite o Columbite o Erythrite o Hemimorphite o Agardite-Y o Augite o Calciohilairite (Ferrocolumbite) o Euchroite o Hercynite o Agate (Quartz) o Aurostibite o Calcite, see also o Conichalcite o Euxenite o Hessite o Aguilarite o Austinite Manganocalcite o Connellite o Euxenite-Y o Heulandite o Aktashite o Onyx o Copiapite o o Autunite o Fairchildite Hexahydrite o Alabandite o Caledonite o Copper o o Awaruite o Famatinite Hibschite o Albite o Cancrinite o Copper-zinc o o Axinite group o Fayalite Hillebrandite o Algodonite o Carnelian (Quartz) o Coquandite o o Azurite o Feldspar group Hisingerite o Allanite o Cassiterite o Cordierite o o Barite o Ferberite Hongshiite o Allanite-Ce o Catapleiite o Corrensite o o Bastnäsite -

Clay Minerals Soils to Engineering Technology to Cat Litter
Clay Minerals Soils to Engineering Technology to Cat Litter USC Mineralogy Geol 215a (Anderson) Clay Minerals Clay minerals likely are the most utilized minerals … not just as the soils that grow plants for foods and garment, but a great range of applications, including oil absorbants, iron casting, animal feeds, pottery, china, pharmaceuticals, drilling fluids, waste water treatment, food preparation, paint, and … yes, cat litter! Bentonite workings, WY Clay Minerals There are three main groups of clay minerals: Kaolinite - also includes dickite and nacrite; formed by the decomposition of orthoclase feldspar (e.g. in granite); kaolin is the principal constituent in china clay. Illite - also includes glauconite (a green clay sand) and are the commonest clay minerals; formed by the decomposition of some micas and feldspars; predominant in marine clays and shales. Smectites or montmorillonites - also includes bentonite and vermiculite; formed by the alteration of mafic igneous rocks rich in Ca and Mg; weak linkage by cations (e.g. Na+, Ca++) results in high swelling/shrinking potential Clay Minerals are Phyllosilicates All have layers of Si tetrahedra SEM view of clay and layers of Al, Fe, Mg octahedra, similar to gibbsite or brucite Clay Minerals The kaolinite clays are 1:1 phyllosilicates The montmorillonite and illite clays are 2:1 phyllosilicates 1:1 and 2:1 Clay Minerals Marine Clays Clays mostly form on land but are often transported to the oceans, covering vast regions. Kaolinite Al2Si2O5(OH)2 Kaolinite clays have long been used in the ceramic industry, especially in fine porcelains, because they can be easily molded, have a fine texture, and are white when fired. -

Part 629 – Glossary of Landform and Geologic Terms
Title 430 – National Soil Survey Handbook Part 629 – Glossary of Landform and Geologic Terms Subpart A – General Information 629.0 Definition and Purpose This glossary provides the NCSS soil survey program, soil scientists, and natural resource specialists with landform, geologic, and related terms and their definitions to— (1) Improve soil landscape description with a standard, single source landform and geologic glossary. (2) Enhance geomorphic content and clarity of soil map unit descriptions by use of accurate, defined terms. (3) Establish consistent geomorphic term usage in soil science and the National Cooperative Soil Survey (NCSS). (4) Provide standard geomorphic definitions for databases and soil survey technical publications. (5) Train soil scientists and related professionals in soils as landscape and geomorphic entities. 629.1 Responsibilities This glossary serves as the official NCSS reference for landform, geologic, and related terms. The staff of the National Soil Survey Center, located in Lincoln, NE, is responsible for maintaining and updating this glossary. Soil Science Division staff and NCSS participants are encouraged to propose additions and changes to the glossary for use in pedon descriptions, soil map unit descriptions, and soil survey publications. The Glossary of Geology (GG, 2005) serves as a major source for many glossary terms. The American Geologic Institute (AGI) granted the USDA Natural Resources Conservation Service (formerly the Soil Conservation Service) permission (in letters dated September 11, 1985, and September 22, 1993) to use existing definitions. Sources of, and modifications to, original definitions are explained immediately below. 629.2 Definitions A. Reference Codes Sources from which definitions were taken, whole or in part, are identified by a code (e.g., GG) following each definition. -

06 11 09 Separation & Preparation of Biotite and Muscovite Samples For
06 11 09 Separation & Preparation of Biotite and Muscovite samples for 40Ar/39Ar analysis Karl Lang When dealing with detrital samples, sample contamination is a big problem. To avoid this at all costs be clean, this means handling samples one at a time and cleaning all equipment entirely after each sample handling. Only handle samples in a quite (i.e. not windy) environment, where there is little chance of spilling or blowing samples away. Handle samples over clean copier paper, and change the paper after each sample. Use compressed air (either canned or from a compressor) to clean all equipment in separation stages and methanol to keep equipment dirt and dust free in the preparation stages. This can be often be tedious work, I recommend finding a good book on tape or podcast to listen to. Estimate times to completion are stated. Detrital Samples Separation I. Sample drying (1-3 days) For detrital samples it is likely that samples will be wet. Simple let the samples dry in the open air, or under a mild lamp on paper plates. It may be necessary to occasionally stir up samples to get them to dry faster, if you do this, clean the stirrer after each sample. Split samples using a riffle splitter to obtain a quantity for the rest of the process, do this in the rock room. II. Sample Sieving (3-7 days) First locate a set of sieves that can be intensively cleaned. There are a set of appropriate sieves in 317 for this use, be careful using other's sieves as you will likely bend the meshes during cleaning. -

Muscovite Solid Solutions in the System K20-Mgo-Feo-A1203-Sio2-H20: an Experimental Study at 2 Kbar Ph2o and Comparison with Natural Li-Free White Micas
MINERALOGICAL MAGAZINE, JUNE 1986, VOL. 50, PP. 257-66 Muscovite solid solutions in the system K20-MgO-FeO-A1203-SiO2-H20: an experimental study at 2 kbar PH2o and comparison with natural Li-free white micas GILLES MoNIER Laboratoire de P&rologie, Universit6 d'Or16ans, 45046 Orlrans Cedex, France AND JI~AN-LouIs ROBERT Centre de Recherche sur la Synthrse et Chimie des MinSraux, G.I.S.C.N.R.S.-B.R.G.M., 1A rue de la Frrollerie, 45071 Odrans Cedex 2, France ABSTRACT. This paper presents the results of an experi- K EY W OR O S : muscovite, phengite, solid solution, crystal- mental study of muscovite solid solutions in the system chemistry, experimental mineralogy, granites, hydro- K20-M~+O-A1203 SiO2-H20 (HF), with M2+= thermal alteration. Mg 2+ or Fe 2§ in the temperature range 300 700~ under 2 kbar P.~o, Muscovite solid solutions can be described, in this system, as the result of two substitutions. NATURAL lithium-free white micas are generally One is the phengitic substitution (x), which preserves the described as solid solutions between the muscovite pure dioctahedral character of the mica; the second is the end member K(A12R)(Si3AI)Olo(OH)2, where [] biotitic substitution (y), which leads to trioctahedral stands for an octahedral vacant site, and the micas and does not change the composition of the celadonite end member K(AIM z+ [3)Si4010(OH)2, tetrahedral layer Si3AI. The general formula of muscovite with M 2 + = Mg 2., Fe E+, thus, they are considered in this system is K(A12_~_2y/aM2+yOl_y/a)(Sia+~All_x) to belong to the so-called phengitic series. -
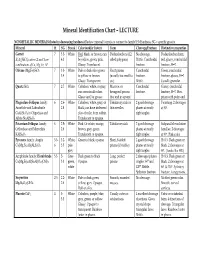
Mineral Identification Chart – LECTURE
Mineral Identification Chart – LECTURE NONMETALLIC MINERALS (listed in decreasing hardness) Review mineral formula to connect to family! H=Hardness; SG = specific gravity Mineral H SG Streak Color (and/or luster) Form Cleavage/Fracture Distinctive properties Garnet 7 3.5- White Red, black, or brown; can Dodecahedrons (12- No cleavage. Dodecahedron form, X3Y2(SiO4)3 where X and Y are 4.3 be yellow, green, pink. sided polygons) Brittle. Conchoidal red, glassy, conchoidal combinations of Ca, Mg, Fe, Al Glassy. Translucent. fracture. fracture, H=7. Olivine (Mg,Fe)2SiO4 7 3.3- White Pale or dark olive green Short prisms Conchoidal Green, conchoidal 3.4 to yellow or brown. (usually too small to fracture. fracture, glassy, H=7. Glassy. Transparent. see). Brittle. Usually granular. Quartz SiO2 7 2.7 White Colorless, white, or gray; Massive; or Conchoidal Glassy, conchoidal can occur in all colors. hexagonal prisms fracture. fracture, H=7. Hex. Glassy and/or greasy. that end in a point. prism with point end. Plagioclase Feldspar family: 6 2.6- White Colorless, white, gray, or Tabular crystals or 2 good cleavage Twinning. 2 cleavages Anorthite and Labradorite 2.8 black; can have iridescent thin needles planes at nearly at 90°. CaAl2Si2O8 to Oligoclase and play of color from within. right angles. Albite NaAlSi3O8 Translucent to opaque. Potassium Feldspar family: 6 2.5- White Pink. Or white, orange, Tabular crystals 2 good cleavage Subparallel exsolution Orthoclase and Microcline 2.6 brown, gray, green. planes at nearly lamellae. 2 cleavages KAlSi3O8 Translucent to opaque. right angles. at 90°. Pink color. Pyroxene family: Augite 5.5- 3.2- White, Green to black; opaque. -

Global Lithium Sources—Industrial Use and Future in the Electric Vehicle Industry: a Review
resources Review Global Lithium Sources—Industrial Use and Future in the Electric Vehicle Industry: A Review Laurence Kavanagh * , Jerome Keohane, Guiomar Garcia Cabellos, Andrew Lloyd and John Cleary EnviroCORE, Department of Science and Health, Institute of Technology Carlow, Kilkenny, Road, Co., R93-V960 Carlow, Ireland; [email protected] (J.K.); [email protected] (G.G.C.); [email protected] (A.L.); [email protected] (J.C.) * Correspondence: [email protected] Received: 28 July 2018; Accepted: 11 September 2018; Published: 17 September 2018 Abstract: Lithium is a key component in green energy storage technologies and is rapidly becoming a metal of crucial importance to the European Union. The different industrial uses of lithium are discussed in this review along with a compilation of the locations of the main geological sources of lithium. An emphasis is placed on lithium’s use in lithium ion batteries and their use in the electric vehicle industry. The electric vehicle market is driving new demand for lithium resources. The expected scale-up in this sector will put pressure on current lithium supplies. The European Union has a burgeoning demand for lithium and is the second largest consumer of lithium resources. Currently, only 1–2% of worldwide lithium is produced in the European Union (Portugal). There are several lithium mineralisations scattered across Europe, the majority of which are currently undergoing mining feasibility studies. The increasing cost of lithium is driving a new global mining boom and should see many of Europe’s mineralisation’s becoming economic. The information given in this paper is a source of contextual information that can be used to support the European Union’s drive towards a low carbon economy and to develop the field of research. -

Cation Ordering and Pseudosymmetry in Layer Silicates'
I A merican M ineralogist, Volume60. pages175-187, 1975 Cation Ordering and Pseudosymmetryin Layer Silicates' S. W. BerI-nv Departmentof Geologyand Geophysics,Uniuersity of Wisconsin-Madison Madison, Wisconsin5 3706 Abstract The particular sequenceof sheetsand layers present in the structure of a layer silicate createsan ideal symmetry that is usually basedon the assumptionsof trioctahedralcompositions, no significantdistor- tion, and no cation ordering.Ordering oftetrahedral cations,asjudged by mean l-O bond lengths,has been found within the constraints of the ideal spacegroup for specimensof muscovite-3I, phengile-2M2, la-4 Cr-chlorite, and vermiculite of the 2-layer s type. Many ideal spacegroups do not allow ordering of tetrahedralcations because all tetrahedramust be equivalentby symmetry.This includesthe common lM micasand chlorites.Ordering oftetrahedral cations within subgroupsymmetries has not beensought very often, but has been reported for anandite-2Or, llb-2prochlorite, and Ia-2 donbassite. Ordering ofoctahedral cations within the ideal spacegroups is more common and has been found for muscovite-37, lepidolite-2M", clintonite-lM, fluoropolylithionite-lM,la-4 Cr-chlorite, lb-odd ripidolite, and vermiculite. Ordering in subgroup symmetries has been reported l-oranandite-2or, IIb-2 prochlorite, and llb-4 corundophilite. Ordering in local out-of-step domains has been documented by study of diffuse non-Bragg scattering for the octahedral catlons in polylithionite according to a two-dimensional pattern and for the interlayer cations in vermiculite over a three-cellsuperlattice. All dioctahedral layer silicates have ordered vacant octahedral sites, and the locations of the vacancies change the symmetry from that of the ideal spacegroup in kaolinite, dickite, nacrite, and la-2 donbassite Four new structural determinations are reported for margarite-2M,, amesile-2Hr,cronstedtite-2H", and a two-layercookeite. -

Cr3+ in Phyllosilicates
Mineral Spectroscopy: A Tribute to Roger G. Bums © The Geochemical Society, Special Publication No.5, ]996 Editors: M. D. Dyar, C. McCammon and M. W. Schaefer 3 Cr + in phyllosilicates: Influence of the nature of coordinating ligands and their next cationic neighbors on the crystal field parameters I 2 2 A. N. PLATONOV , K. LANGER , M. ANDRUT .3, G. CALAS4 'Institute of Geochemistry, Mineralogy and Ore Formation, Academy of Science of Ukraine, 252680 Kiev, Ukraine 2Institute of Mineralogy and Crystallography, Technical University, D-10623 Berlin, Germany 3GeoForschungszentrum Potsdam, D-14473 Potsdam, Deutschland "Laboratoire de Mineralogie et de Cristallographie, Universite de Paris 6 et 7, F-7525l Paris, France 3 Abstract- The electronic absorption spectra of Cr + -bearing clinochlore (I, kammererite), amesite (II), muscovite (III, fuchsite), dickite (IV), and montmorillonite (V, volkonskite) analysed by electron microprobe were obtained on single crystals. Microscope-spectrometric techniques and polarized radiation in the spectral range 10000-38000 cm " (I, II, III) or (on fine grained material) diffuse reflectance spectrometry in the spectral range 8000-50000 cm-I (IV, V) were used. The ligand field theoretical evaluation of the spectra showed the following: (i) The fl.o = 10Dq = f(1/R5) relation, wherein fl.o is the octahedral crystal field parameter and R the mean cation ligand distance, is valid within each series of layer silicates containing octahedral Cr3+ either in a trioctahedral layer (I, II and phlogopite) or in a dioctahedral layer (III, IV, V). Between the two functions, fl.o.trioct = f(1lR~ioct) and fl.o.di=t = f(1/R~ioct), there exists an energy difference of about 2200 em -I. -

A RARE-ALKALI BIOTITE from KINGS MOUNTAIN, NORTH CAROLINA1 Fnanr L
A RARE-ALKALI BIOTITE FROM KINGS MOUNTAIN, NORTH CAROLINA1 FnaNr L. Hnss2 arqn Ror-r.rx E. SrrvrNs3 Severalyears ago, after Judge Harry E. Way of Custer, South Dakota, had spectroscopically detected the rare-alkali metals in a deep-brown mica from a pegmatite containing pollucite and lithium minerals, in Tin Mountain, 7 miles west of Custer, another brown mica was collected, which had developed notably in mica schist at its contact with a similar mass of pegmatite about one half mile east of Tin Mountai". J. J. Fahey of the United States GeolgoicalSurvey analyzed the mica, and it proved to contain the rare-alkali metalsaand to be considerably difierent from any mica theretofore described. Although the cesium-bearing minerals before known (pollucite, lepidolite, and beryl) had come from the zone of highest temperature in the pegmatite, the brown mica was from the zone of lowest temperature. The occurrence naturally suggestedthat where dark mica was found developed at the border of a pegmatite, especially one carrying lithium minerals, it should be examined for the rare-alkali metals. As had been found by Judge Way, spectroscopictests on the biotite from Tin Moun- tain gave strong lithium and rubidium lines, and faint cesium lines. Lithium lines were shown in a biotite from the border of the Morefield pegmatite, a mile south of Winterham, Virginia, but rubidium and cesium w'erenot detected. $imilarly placed dark micas from Newry and Hodgeon HiII, near Buckfield, Maine, gave negative results. They should be retested. Tests by Dr. Charles E. White on a shiny dark mica from the Chestnut FIat pegmatite near Spruce Pine, North Carolina, gave strong lithium and weaker cesium lines.