Abstracts Book Igc 2015 (Pdf)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
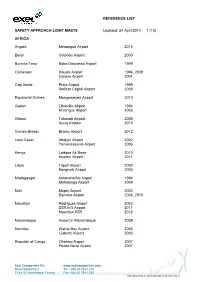
Reference List Safety Approach Light Masts
REFERENCE LIST SAFETY APPROACH LIGHT MASTS Updated: 24 April 2014 1 (10) AFRICA Angola Menongue Airport 2013 Benin Cotonou Airport 2000 Burkina Faso Bobo Diaulasso Airport 1999 Cameroon Douala Airport 1994, 2009 Garoua Airport 2001 Cap Verde Praia Airport 1999 Amilcar Capral Airport 2008 Equatorial Guinea Mongomeyen Airport 2010 Gabon Libreville Airport 1994 M’vengue Airport 2003 Ghana Takoradi Airport 2008 Accra Kotoka 2013 Guinea-Bissau Bissau Airport 2012 Ivory Coast Abidjan Airport 2002 Yamoussoukro Airport 2006 Kenya Laikipia Air Base 2010 Kisumu Airport 2011 Libya Tripoli Airport 2002 Benghazi Airport 2005 Madagasgar Antananarivo Airport 1994 Mahajanga Airport 2009 Mali Moptu Airport 2002 Bamako Airport 2004, 2010 Mauritius Rodrigues Airport 2002 SSR Int’l Airport 2011 Mauritius SSR 2012 Mozambique Airport in Mozambique 2008 Namibia Walvis Bay Airport 2005 Lüderitz Airport 2005 Republic of Congo Ollombo Airport 2007 Pointe Noire Airport 2007 Exel Composites Plc www.exelcomposites.com Muovilaaksontie 2 Tel. +358 20 754 1200 FI-82110 Heinävaara, Finland Fax +358 20 754 1330 This information is confidential unless otherwise stated REFERENCE LIST SAFETY APPROACH LIGHT MASTS Updated: 24 April 2014 2 (10) Brazzaville Airport 2008, 2010, 2013 Rwanda Kigali-Kamombe International Airport 2004 South Africa Kruger Mpumalanga Airport 2002 King Shaka Airport, Durban 2009 Lanseria Int’l Airport 2013 St. Helena Airport 2013 Sudan Merowe Airport 2007 Tansania Dar Es Salaam Airport 2009 Tunisia Tunis–Carthage International Airport 2011 ASIA China -

Mr. Linas LINKEVIČIUS Užsienio Reikalų Ministerija J. Tumo-Vaižganto G
EUROPEAN COMMISSION PUBLIC VERSION This document is made available for information purposes only. Brussels, 25.08.2016 C(2016) 5375 final Subject: State aid SA.40197 (2016/N) – Lithuania Investment in infrastructure at Vilnius airport Sir, 1 PROCEDURE (1) On 31 December 2015, the Lithuanian authorities notified a measure concerning the modernisation of the Vilnius Airport's infrastructure (hereinafter “the measure”) after pre-notification contacts. (2) Between January and April 2016 several formal and informal contacts and information exchanges have taken place. The reply of the Lithuanian authorities to the Commission services' requests for information was received on 2 June 2016 and an update was received on 21 July 2016. 2 DESCRIPTION OF THE MEASURE 2.1 The beneficiary Vilnius airport/Lietuvos Oro Uostai (3) Since 1 July 2014, the operation of the three Lithuanian airports Vilnius, Kaunas and Palanga has been entrusted to the State-owned company Lietuvos Oro Uostai (LUO) ("Lithuanian Airports") under the control of the Ministry of Transport and Communications. The airport management company ensures account separation for the different airports. Mr. Linas LINKEVIČIUS Užsienio Reikalų Ministerija J. Tumo-Vaižganto g. 2 LT-01511 Vilnius LIETUVOS RESPUBLIKA Commission européenne/Europese Commissie, 1049 Bruxelles/Brussel, BELGIQUE/BELGIË - Tel. +32 22991111 (4) LOU is the direct beneficiary of the notified measure. (5) The three airports complement each other in destinations covered, airlines present and passenger groups served. Vilnius airport is the main capital gateway for business and tourist travel from and to the country, Kaunas airport is focused on facilitating travel at low cost of Lithuanian nationals working/living abroad and Palanga airport provides access to the Scandinavian region using premium Scandinavian airlines for tourists and for business to and around the port city of Klaipeda. -

Lithuanian Airports' Network Incentive Scheme
LITHUANIAN AIRPORTS’ NETWORK INCENTIVE SCHEME Valid from 1 March 2018 CONTENTS AND INFORMATION 1. PREAMBLE……………………………………………………………………………………2 2. DEFINITIONS ............................................................................................................................ 2 3. NETWORK INCENTIVE SCHEME ......................................................................................... 3 3.1. Airport Charges .................................................................................................................... 3 3.1.1. Level of Network Support ............................................................................................ 4 3.1.2. Eligibility ...................................................................................................................... 5 4. DECISIONS ON ELIGIBILITY OF ROUTES AND OTHER CONDITIONS FOR PROVISION OF SUPPORT .......................................................................................................... 5 4.1. Application ........................................................................................................................... 5 4.2. Approval .............................................................................................................................. 6 4.3. Application of Discount ....................................................................................................... 6 5. AMENDMENT OR TERMINATION OF THE SCHEME ....................................................... 6 Appendix 1 ..................................................................................................................................... -

GETTING to KLAIPEDA Flights the Closest Airport – Palanga
GETTING TO KLAIPEDA Flights The closest airport – Palanga International Airport is located on the Northern boundary of Klaipeda , within a 30 min. drive. The airport and Klaipeda are connected by the internationally significant road that extends from Liepaja (Latvia) to Klaipeda. ******************************************************************************** There are Wizzair, Ryanair, Finnair, Brussels Airlines, Austrian Airlines, Aeroflot, Ukrainian International Airlines, LOT, Air Baltics flights to and from Vilnius airport (VNO). Flights map: https://www.vilnius-airport.lt/en/planning-a-trip/flight-map/ There is a special train "Vilnius-Airport" running regularly to Vilnius railway station. The train shuttles 16 times a day, it takes 7 minutes to arrive the railway station from airport. The ticket costs EUR 0.70. It can be purchased on-line and in the train. Next to the airport passenger terminal, there is a railway stop, stairs and an elevator for passengers as well as lighting and passenger safety surveillance camera. There is a footway leading from the railway stop to the airport terminal for passengers; the footway has an installed gallery (it is illuminated during the dark time of the day).www.traukiniobilietas.lt From Vilnius railway station there are 5 trains to Klaipeda every day: at 5:10 (only on working days), 6:50, 9:50, 16:45, 17:40. https://www.traukiniobilietas.lt/portal/routes-schedules You can go to Klaipėda from Vilnius airport by Vilnius airport express bus. https://www.ollex.lt/en/express ******************************************************************************** A lot of Ryanair and Wizzair flyghts are operating to and from Kaunas Airport (KUN). https://www.kaunas-airport.lt/index.php?lang=en&page=destination_map An express bus service runs from Kaunas Airport to Klaipeda and from Klaipeda to Kaunas Airport. -

Airport Integration Study
Rail Baltica Project Airport Integration Study (Passengers and Luggage Services) Feasibility study – Final Report Date 18/10/2019 Final version The sole responsibility of this publication lies with the author. The European Union is not responsible for any use that may be made of the information contained therein. Airport integration study (Passenger and Luggage Services) Table of Contents Rationale ........................................................................................................................................................................................................ 3 Assignment Order identification ..................................................................................................................................................... 3 Brief description of the assignment ............................................................................................................................................... 3 Documents considered for this assignment ............................................................................................................................... 6 List of abbreviations ............................................................................................................................................................................. 8 Definitions ................................................................................................................................................................................................ 9 WP 4: Recommendations -

LIST of REFERENCES ITW GSE 400 Hz Gpus AIRPORTS
Page 1 of 15 January 2017 LIST OF REFERENCES ITW GSE 400 Hz GPUs AIRPORTS Alger Airport Algeria 2005 Zvartnots Airport Armenia 2007 Brisbane Airport Australia 2013 Melbourne Airport Australia 2011-14 Perth Airport Australia 2011-12-13 Klagenfurt Airport Austria 1993 Vienna International Airport Austria 1995-2001-14-15 Bahrain International Airport Bahrain 2010-12 Minsk Airport Belarus 2014 Brussels International Airport Belgium 2001-02-08-15-16 Charleroi Airport Belgium 2006 Sofia Airport Bulgaria 2005 Air Burkina Burkina Faso 2004 Punta Arenas Chile 2001 Santiago Airport Chile 2011 Pointe Noitre Airport Congo Brazzaville 2009-10 Dubrovnik Airport Croatia 2014-16 La Habana Airport Cuba 2010 Larnaca Airport Cyprus 2008 Ostrava Airport Czech Republic 2010 Prague Airport Czech Republic 1996-97-2002-04-05-07-12-14-16 Aalborg Airport Denmark 1997-98-99-2012-15 Billund Airport Denmark 1999-2000-02-08-12-13-16 Copenhagen Airports Authorities Denmark 89-93-99-2000-01-03-07-09-10-11-12-13-14-15-16 Esbjerg Airport Denmark 2007-08-14 Hans Christian Andersen Airport (Odense) Denmark 1991-95-2015 Roenne Airport Denmark 1993 Karup Airport Denmark 1997-2016 Curacao Airport Dutch Antilles 2007 Cairo Intl. Airport Egypt 2015 Tallinn Airport Estonia 2004-05-14 Aéroport de Malabo Equatorial Guinea 2012 Vága Floghavn Faroe Islands 2015 Helsinki-Vantaa Airport Finland 1996-97-2000-05-06-09-10-13-14 Rovaniemi Airport Finland 2000 Turku Airport Finland 2014 Aéroport d’Aiglemont for Prince Aga Khan France 20007 Aéroport de Biarritz France 2009 Aéroport de Brest -

Voorwaarden-Lithuania.Pdf
Country Level Rental Policies Lithuania - LT There will be an additional charge of 6.61 Euro per day plus VAT for each additional authorized driver. Full rental Additional Driver conditions apply to all additional drivers. All renters and additional drivers must be 19 or older. Only renters aged 21 or older can rent the Luxury Van vehicle group. Age Requirements There is no young driver fee. Maximum renting age is 75. Visa, MasterCard and American Express credit cards are accepted as payment for rentals and upon reservation. Cash payments may be accepted in exception to the general rule if a supporting credit card is provided. Cash payments must be arranged and approved by the Station Manager. Deposits: Customers should present valid credit card in his/her own name for security deposit authorization purposes. The Forms of Payment deposit amount will cover the amount of Collision Damage Waiver cover which ranges from 400 to 700 Euro depending upon vehicle class and the cost of a full tank of fuel. The deposit is blocked during the rental period and unblocked after the rental vehicle has been returned in the same condition at the time of rental pickup (no damage, full tank of fuel and no traffic fines or penalties). All drivers (renter and additional drivers) must produce a valid driving license at the time of rental. In addition to a valid driving license, the primary renter must also produce two proofs of address, additional photo identification in the form ID card or passport and a major credit card in his/her own name. -

LSSIP 2019 - LITHUANIA LOCAL SINGLE SKY IMPLEMENTATION Level2019 1 - Implementation Overview
EUROCONTROL LSSIP 2019 - LITHUANIA LOCAL SINGLE SKY IMPLEMENTATION Level2019 1 - Implementation Overview Document Title LSSIP Year 2019 for Lithuania Info Centre Reference 20/01/15/24 Date of Edition 06/04/2020 LSSIP Focal Point Mrs. Julija ČIŽIENĖ, [email protected] SE “Oro navigacija” LSSIP Contact Person Igor MARCETIC, [email protected] EUROCONTROL/NMD/INF/PAS LSSIP Support Team [email protected] Status Released Intended for Agency Stakeholders Available in https://www.eurocontrol.int/service/local-single-sky- implementation-monitoring Reference Documents LSSIP Documents https://www.eurocontrol.int/service/local-single-sky- implementation-monitoring Master Plan Level 3 – Plan Edition https://www.eurocontrol.int/publication/european-atm- 2019 master-plan-implementation-plan-level-3-2019 Master Plan Level 3 – Report Year https://www.eurocontrol.int/publication/european-atm- 2019 master-plan-implementation-report-level-3-2019 European ATM Portal https://www.atmmasterplan.eu/ STATFOR Forecasts https://www.eurocontrol.int/statfor National AIP https://www.ans.lt/en/ais/aip-extracts/ FAB Performance Plan http://www.balticfab.eu/uploads/files/dir5/9_0.php LSSIP Year 2019 Lithuania – Level 1 Released issue APPROVAL SHEET The following authorities have approved all parts of the LSSIP Year 2019 document and the signatures confirm the correctness of the reported information and reflect the commitment to implement the actions laid down in the European ATM Master Plan Level 3 (Implementation View) – Edition 2019. Stakeholder -

AIRPORT DEVELOPMENT International News Each Issue of Airport Development Focuses on a Different Region of the World, with Global News at the End of This Section
Page 1 of 12 25 April 2020 No. 1119 DEV Published biweekly – available by annual subscription only – www.mombergerairport.info Editorial office / Subscriptions Phone: +1 519 833 4642 e-mail: [email protected] Managing Editor / Publisher: Martin Lamprecht [email protected] News Editor: Paul Ellis [email protected] – Founding Editor: Manfred Momberger Momberger Airport Information by Air Trans Source Inc. – international news & data – published since 1973 AIRPORT DEVELOPMENT International News Each issue of Airport Development focuses on a different region of the world, with global news at the end of this section. A list of past focus regions published in recent years can be downloaded from the Bonus section in the subscriber pages of our website. Focus Region: Central Europe - 1 LITHUANIA Vilnius International Airport had planned a major expansion, including the construction of a new terminal to serve increasing passenger traffic. The proposed Departures terminal would boost the airport’s operational and technological capabilities, while improving the range of non-aviation services. The airport expansion project was scheduled to start in the second half of 2020 and was expected to be completed in the second half of 2022. A contractor to undertake the construction was expected to be selected by mid-2020. The new terminal would be a two-storey building with over 14,400 m² of space. The total area of the passenger terminal was expected to increase by 33% and the passenger-handling capacity would grow from 1,200 per hour to 2,400 passengers upon completion of the project. The terminal would be situated between the existing passenger terminal and a new VIP terminal, already under construction. -

Covid-19 Global Travel Restrictions 20 November 2020 17:00 UTC
Covid-19 Global Travel Restrictions 20 November 2020 17:00 UTC Below you will find useful information regarding every country around the world where travel restrictions or other conditions are currently in effect. To search for a country of interest, either simply scroll through the document or click Find within the Edit area of the PDF menu ribbon and type the first few letters of the country name you are looking for. Countries highlighted in yellow represent those with updates since our last report. Country Travel Restrictions and/or Special Conditions Afghanistan International • The Afghanistan Civil Aviation Authority has announced that reduced international commercial flights are gradually resuming. Emirates, Turkish, Ariana, and Kam airlines are resuming operations. • All passengers travelling with Emirates and Fly Dubai from Afghanistan must be able to present a COVID-19 negative certificate to be accepted for travel. This certificate must be issued no more than 96 hours prior to commencing your travel by a laboratory accredited to issue test certificates. • Land borders with China, Tajikistan, Turkmenistan, and Uzbekistan are shut, although cargo and other essential traffic is exempt. • The border with Iran is open. • Pakistan has reopened its border with the country for trade. • Arriving passengers who do not show COVID-19 symptoms are advised to quarantine for 14 days. Those with symptoms should contact the Ministry of Public Health for care. Domestic • Facemasks are mandatory in public places. • Domestic flights are currently operating on a very limited basis. © 2020 Anvil Group Confidential • Movement between Kabul and the other provinces is restricted, although the movement of essential goods and medical professionals are exempt. -

ARRIVAL and ACCOMMODATION Also Available On
IASIA-KTU conference CORRUPTION, ETHICS AND CULTURE IN PUBLIC ADMINISTRATION 24-25 April 2019, Kaunas, Lithuania ARRIVAL AND ACCOMMODATION Also available on: https://iasiaconference.ktu.edu/#Accommodation ARRIVING BY PLANE Participants may fly to and from Kaunas and Vilnius airports. Traveling to and from Kaunas airport (in Lithuanian – Kauno oro uostas) Bus No. 29 connects Kaunas International Airport with the city centre. The journey takes about 45 minutes with the bus timetable being coordinated with flight schedules. A taxi service is available for those who need a speedier transfer. The taxi can be hailed on the streets, called by phone or using mobile App. The average price for one kilometer by taxi is 0,70 EUR or you can use Taxify in Kaunas. Wherever you are in the beautiful city of Kaunas, from Defensive medieval Kaunas Castle to Town Hall Square at the heart of the Old Town Kaunas, Taxify is just a tap of a button away. Traveling to and from Vilnius airport (in Lithuanian – Vilniaus oro uostas). From Vilnius Airport there are frequent train and bus connections to Kaunas (please also see below arriving by train and arriving by bus).The journey takes about 1-1,5 hours. There are several ways to get from Vilnius Airport (VNO) to Kaunas: by bus, train or car. Vilnius Airport is located approximately 100 km from Kaunas and it takes approx. 1 hour to travel by car. By intercity bus or train it takes 1-1,5 hour at a price of 6-10 euros. Bus tickets and schedule: https://www.autobusubilietai.lt Train tickets: http://www.litrail.lt/en/home You can reach bus and train stations in Vilnius from the airport by taxi at a cost of 4-5 eur (see the route here) or by bus No. -

Airport Lounge List
Dreamfolks Membership Programme: Airport Lounge List Last updated on 21th Jan, 2021 S.No Country City Region Airport Name Lounge Name Terminal United Arab Middle International 1 Dubai Dubai International Marhaba Lounge Emirates East Departure Terminal 3 United Arab Middle International 2 Dubai Dubai International Marhaba lounge Emirates East Departure Terminal 1 United Arab Middle International 3 Dubai Dubai International Marhaba Lounge (T2) Emirates East Departure Terminal 2 South 4 Singapore Singapore Singapore Changi International SATS Premier Lounge (T2) Terminal 2 East Asia South 5 Singapore Singapore Singapore Changi International SATS Premier Lounge Terminal 3 East Asia South Miracle First Class 6 Thailand Bangkok Bangkok Suvarnabhumi International International East Asia Lounge(Concourse A - Level 3) International United Arab Middle Miracle First Class 7 Dubai Dubai International Airport-DXB Departure Terminal 3 Emirates East Lounge(Concourse A - Level 3) Concourse B Middle Miracle First Class 8 Qatar Doha Doha Hamad International International East Lounge(Concourse A - Level 3) South Miracle First Class International 9 Thailand Bangkok Suvarnabhumi International Airport East Asia Lounge(Concourse A - Level 3) Departures International South Miracle First Class 10 Sri Lanka Colombo Bandaranaike International Airport Departure Main East Asia Lounge(Concourse A - Level 3) Terminal South Miracle First Class 11 Thailand Bangkok Bangkok Don Mueang International Terminal 1 East Asia Lounge(Concourse A - Level 3) South Miracle First Class