Lecture 9 Point Group Symmetries Elena Orlova
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In Order for the Figure to Map Onto Itself, the Line of Reflection Must Go Through the Center Point
3-5 Symmetry State whether the figure appears to have line symmetry. Write yes or no. If so, copy the figure, draw all lines of symmetry, and state their number. 1. SOLUTION: A figure has reflectional symmetry if the figure can be mapped onto itself by a reflection in a line. The figure has reflectional symmetry. In order for the figure to map onto itself, the line of reflection must go through the center point. Two lines of reflection go through the sides of the figure. Two lines of reflection go through the vertices of the figure. Thus, there are four possible lines that go through the center and are lines of reflections. Therefore, the figure has four lines of symmetry. eSolutionsANSWER: Manual - Powered by Cognero Page 1 yes; 4 2. SOLUTION: A figure has reflectional symmetry if the figure can be mapped onto itself by a reflection in a line. The given figure does not have reflectional symmetry. There is no way to fold or reflect it onto itself. ANSWER: no 3. SOLUTION: A figure has reflectional symmetry if the figure can be mapped onto itself by a reflection in a line. The given figure has reflectional symmetry. The figure has a vertical line of symmetry. It does not have a horizontal line of symmetry. The figure does not have a line of symmetry through the vertices. Thus, the figure has only one line of symmetry. ANSWER: yes; 1 State whether the figure has rotational symmetry. Write yes or no. If so, copy the figure, locate the center of symmetry, and state the order and magnitude of symmetry. -

Molecular Symmetry
Molecular Symmetry Symmetry helps us understand molecular structure, some chemical properties, and characteristics of physical properties (spectroscopy) – used with group theory to predict vibrational spectra for the identification of molecular shape, and as a tool for understanding electronic structure and bonding. Symmetrical : implies the species possesses a number of indistinguishable configurations. 1 Group Theory : mathematical treatment of symmetry. symmetry operation – an operation performed on an object which leaves it in a configuration that is indistinguishable from, and superimposable on, the original configuration. symmetry elements – the points, lines, or planes to which a symmetry operation is carried out. Element Operation Symbol Identity Identity E Symmetry plane Reflection in the plane σ Inversion center Inversion of a point x,y,z to -x,-y,-z i Proper axis Rotation by (360/n)° Cn 1. Rotation by (360/n)° Improper axis S 2. Reflection in plane perpendicular to rotation axis n Proper axes of rotation (C n) Rotation with respect to a line (axis of rotation). •Cn is a rotation of (360/n)°. •C2 = 180° rotation, C 3 = 120° rotation, C 4 = 90° rotation, C 5 = 72° rotation, C 6 = 60° rotation… •Each rotation brings you to an indistinguishable state from the original. However, rotation by 90° about the same axis does not give back the identical molecule. XeF 4 is square planar. Therefore H 2O does NOT possess It has four different C 2 axes. a C 4 symmetry axis. A C 4 axis out of the page is called the principle axis because it has the largest n . By convention, the principle axis is in the z-direction 2 3 Reflection through a planes of symmetry (mirror plane) If reflection of all parts of a molecule through a plane produced an indistinguishable configuration, the symmetry element is called a mirror plane or plane of symmetry . -

The Cubic Groups
The Cubic Groups Baccalaureate Thesis in Electrical Engineering Author: Supervisor: Sana Zunic Dr. Wolfgang Herfort 0627758 Vienna University of Technology May 13, 2010 Contents 1 Concepts from Algebra 4 1.1 Groups . 4 1.2 Subgroups . 4 1.3 Actions . 5 2 Concepts from Crystallography 6 2.1 Space Groups and their Classification . 6 2.2 Motions in R3 ............................. 8 2.3 Cubic Lattices . 9 2.4 Space Groups with a Cubic Lattice . 10 3 The Octahedral Symmetry Groups 11 3.1 The Elements of O and Oh ..................... 11 3.2 A Presentation of Oh ......................... 14 3.3 The Subgroups of Oh ......................... 14 2 Abstract After introducing basics from (mathematical) crystallography we turn to the description of the octahedral symmetry groups { the symmetry group(s) of a cube. Preface The intention of this account is to provide a description of the octahedral sym- metry groups { symmetry group(s) of the cube. We first give the basic idea (without proofs) of mathematical crystallography, namely that the 219 space groups correspond to the 7 crystal systems. After this we come to describing cubic lattices { such ones that are built from \cubic cells". Finally, among the cubic lattices, we discuss briefly the ones on which O and Oh act. After this we provide lists of the elements and the subgroups of Oh. A presentation of Oh in terms of generators and relations { using the Dynkin diagram B3 is also given. It is our hope that this account is accessible to both { the mathematician and the engineer. The picture on the title page reflects Ha¨uy'sidea of crystal structure [4]. -

Chapter 1 – Symmetry of Molecules – P. 1
Chapter 1 – Symmetry of Molecules – p. 1 - 1. Symmetry of Molecules 1.1 Symmetry Elements · Symmetry operation: Operation that transforms a molecule to an equivalent position and orientation, i.e. after the operation every point of the molecule is coincident with an equivalent point. · Symmetry element: Geometrical entity (line, plane or point) which respect to which one or more symmetry operations can be carried out. In molecules there are only four types of symmetry elements or operations: · Mirror planes: reflection with respect to plane; notation: s · Center of inversion: inversion of all atom positions with respect to inversion center, notation i · Proper axis: Rotation by 2p/n with respect to the axis, notation Cn · Improper axis: Rotation by 2p/n with respect to the axis, followed by reflection with respect to plane, perpendicular to axis, notation Sn Formally, this classification can be further simplified by expressing the inversion i as an improper rotation S2 and the reflection s as an improper rotation S1. Thus, the only symmetry elements in molecules are Cn and Sn. Important: Successive execution of two symmetry operation corresponds to another symmetry operation of the molecule. In order to make this statement a general rule, we require one more symmetry operation, the identity E. (1.1: Symmetry elements in CH4, successive execution of symmetry operations) 1.2. Systematic classification by symmetry groups According to their inherent symmetry elements, molecules can be classified systematically in so called symmetry groups. We use the so-called Schönfliess notation to name the groups, Chapter 1 – Symmetry of Molecules – p. 2 - which is the usual notation for molecules. -

Pose Estimation for Objects with Rotational Symmetry
Pose Estimation for Objects with Rotational Symmetry Enric Corona, Kaustav Kundu, Sanja Fidler Abstract— Pose estimation is a widely explored problem, enabling many robotic tasks such as grasping and manipulation. In this paper, we tackle the problem of pose estimation for objects that exhibit rotational symmetry, which are common in man-made and industrial environments. In particular, our aim is to infer poses for objects not seen at training time, but for which their 3D CAD models are available at test time. Previous X 2 X 1 X 2 X 1 work has tackled this problem by learning to compare captured Y ∼ 2 Y ∼ 2 Y ∼ 2 Y ∼ 2 views of real objects with the rendered views of their 3D CAD Z ∼ 6 Z ∼ 1 Z ∼ Z ∼ 1 ∼ ∼ ∼ ∞ ∼ models, by embedding them in a joint latent space using neural Fig. 1. Many industrial objects such as various tools exhibit rotational networks. We show that sidestepping the issue of symmetry symmetries. In our work, we address pose estimation for such objects. in this scenario during training leads to poor performance at test time. We propose a model that reasons about rotational symmetry during training by having access to only a small set of that this is not a trivial task, as the rendered views may look symmetry-labeled objects, whereby exploiting a large collection very different from objects in real images, both because of of unlabeled CAD models. We demonstrate that our approach different background, lighting, and possible occlusion that significantly outperforms a naively trained neural network on arise in real scenes. -

Symmetry of Graphs. Circles
Symmetry of graphs. Circles Symmetry of graphs. Circles 1 / 10 Today we will be interested in reflection across the x-axis, reflection across the y-axis and reflection across the origin. Reflection across y reflection across x reflection across (0; 0) Sends (x,y) to (-x,y) Sends (x,y) to (x,-y) Sends (x,y) to (-x,-y) Examples with Symmetry What is Symmetry? Take some geometrical object. It is called symmetric if some geometric move preserves it Symmetry of graphs. Circles 2 / 10 Reflection across y reflection across x reflection across (0; 0) Sends (x,y) to (-x,y) Sends (x,y) to (x,-y) Sends (x,y) to (-x,-y) Examples with Symmetry What is Symmetry? Take some geometrical object. It is called symmetric if some geometric move preserves it Today we will be interested in reflection across the x-axis, reflection across the y-axis and reflection across the origin. Symmetry of graphs. Circles 2 / 10 Sends (x,y) to (-x,y) Sends (x,y) to (x,-y) Sends (x,y) to (-x,-y) Examples with Symmetry What is Symmetry? Take some geometrical object. It is called symmetric if some geometric move preserves it Today we will be interested in reflection across the x-axis, reflection across the y-axis and reflection across the origin. Reflection across y reflection across x reflection across (0; 0) Symmetry of graphs. Circles 2 / 10 Sends (x,y) to (-x,y) Sends (x,y) to (x,-y) Sends (x,y) to (-x,-y) Examples with Symmetry What is Symmetry? Take some geometrical object. -

12-5 Worksheet Part C
Name ________________________________________ Date __________________ Class__________________ LESSON Reteach 12-5 Symmetry A figure has symmetry if there is a transformation of the figure such that the image and preimage are identical. There are two kinds of symmetry. The figure has a line of symmetry that divides the figure into two congruent halves. Line Symmetry one line of symmetry two lines of symmetry no line symmetry When a figure is rotated between 0° and 360°, the resulting figure coincides with the original. • The smallest angle through which the figure is rotated to coincide with itself is called the angle of rotational symmetry. • The number of times that you can get an identical figure when repeating the degree of rotation is called the order of the rotational Rotational symmetry. Symmetry angle: 180° 120° no rotational order: 2 3 symmetry Tell whether each figure has line symmetry. If so, draw all lines of symmetry. 1. 2. _________________________________________ ________________________________________ Tell whether each figure has rotational symmetry. If so, give the angle of rotational symmetry and the order of the symmetry. 3. 4. _________________________________________ ________________________________________ Original content Copyright © by Holt McDougal. Additions and changes to the original content are the responsibility of the instructor. 12-38 Holt Geometry Name ________________________________________ Date __________________ Class__________________ LESSON Reteach 12-5 Symmetry continued Three-dimensional figures can also have symmetry. Symmetry in Three Description Example Dimensions A plane can divide a figure into two Plane Symmetry congruent halves. There is a line about which a figure Symmetry About can be rotated so that the image and an Axis preimage are identical. -

Symmetry and Groups, and Crystal Structures
CHAPTER 3: SYMMETRY AND GROUPS, AND CRYSTAL STRUCTURES Sarah Lambart RECAP CHAP. 2 2 different types of close packing: hcp: tetrahedral interstice (ABABA) ccp: octahedral interstice (ABCABC) Definitions: The coordination number or CN is the number of closest neighbors of opposite charge around an ion. It can range from 2 to 12 in ionic structures. These structures are called coordination polyhedron. RECAP CHAP. 2 Rx/Rz C.N. Type Hexagonal or An ideal close-packing of sphere 1.0 12 Cubic for a given CN, can only be Closest Packing achieved for a specific ratio of 1.0 - 0.732 8 Cubic ionic radii between the anions and 0.732 - 0.414 6 Octahedral Tetrahedral (ex.: the cations. 0.414 - 0.225 4 4- SiO4 ) 0.225 - 0.155 3 Triangular <0.155 2 Linear RECAP CHAP. 2 Pauling’s rule: #1: the coodination polyhedron is defined by the ratio Rcation/Ranion #2: The Electrostatic Valency (e.v.) Principle: ev = Z/CN #3: Shared edges and faces of coordination polyhedra decreases the stability of the crystal. #4: In crystal with different cations, those of high valency and small CN tend not to share polyhedral elements #5: The principle of parsimony: The number of different sites in a crystal tends to be small. CONTENT CHAP. 3 (2-3 LECTURES) Definitions: unit cell and lattice 7 Crystal systems 14 Bravais lattices Element of symmetry CRYSTAL LATTICE IN TWO DIMENSIONS A crystal consists of atoms, molecules, or ions in a pattern that repeats in three dimensions. The geometry of the repeating pattern of a crystal can be described in terms of a crystal lattice, constructed by connecting equivalent points throughout the crystal. -

Download English-US Transcript (PDF)
MITOCW | ocw-3.60-13oct2005-pt2-220k_512kb.mp4 PROFESSOR: To mention, most everyone did extremely well on the quiz. But I sense that there's still some of you who have not yet come to terms with crystallographic directions and planes, and you feel a little bit awkward in distinguishing brackets around the HKL and parentheses around HKL. And there are some people who generally get that straightened out, but when I said point group, suddenly pictures of lattices with fourfold axes and twofold axes adorning them came in, and that isn't involved in a point group at also. Again, a point group the symmetry about point. A space group is symmetry spread out through all of space and infinite numbers. So let me say a little bit about resources. I don't know whether you've been following what we've been doing in the notes from Buerger's book that I passed out. That was hard to do is because we did the plane groups, and he doesn't touch them at all. So now we're back following once again Buerger's treatment quite closely. So read the book. And if you like, I can tell you with the end of each lecture, this stuff is on pages 57 through 62. The other thing. As you'll notice, this nonintrusive gentleman in the back is making videotapes of all the lectures. These are eventually going to go up on the website as OpenCourseWare. We were just speaking about that, and it takes a while before they get up, but I have a disk of every lecture. -

Math 203 Lecture 12 Notes: Tessellation – a Complete Covering
Math 103 Lecture #12 class notes page 1 Math 203 Lecture 12 notes: Note: this lecture is very “visual,” i.e., lots of pictures illustrating tessellation concepts Tessellation – a complete covering of a plane by one or more figures in a repeating pattern, with no overlapping. There are only 3 regular polygons that can tessellate a plane without being combined with other polygons: 1) equilateral triangle 2)square 3) regular hexagon. Once a basic design has been constructed, it may be extended through 3 different transformations: translation (slide), rotation (turn), or reflection (flip). FLIP: A figure has line symmetry if there is a line of reflection such that each point in the figure can be reflected into the figure. Line symmetry is sometimes referred to as mirror symmetry, axial, symmetry, bilateral symmetry, or reflective symmetry. The line of symmetry may be vertical, horizontal, or slanted. A figure may have one or more lines of symmetry, or none. Regular polygons with n sides have n lines of symmetry. Any line through the center of a circle is a line of symmetry for a circle. The circle is the most symmetric of all plane figures. The musical counterpart of horizontal reflection (across the y-axis) is called retrogression, while vertical reflection (across the x-axis) is called inversion. SLIDE: Translation (or slide translation) is one in which a figure moves without changing size or shape. It can be thought of as a motion that moves the figure from one location in a plane to another. The distance that the figure moves, or slides, is called the magnitude. -

Crystallography: Symmetry Groups and Group Representations B
EPJ Web of Conferences 22, 00006 (2012) DOI: 10.1051/epjconf/20122200006 C Owned by the authors, published by EDP Sciences, 2012 Crystallography: Symmetry groups and group representations B. Grenier1 and R. Ballou2 1SPSMS, UMR-E 9001, CEA-INAC / UJF-Grenoble, MDN, 38054 Grenoble, France 2Institut Néel, CNRS / UJF, 25 rue des Martyrs, BP. 166, 38042 Grenoble Cedex 9, France Abstract. This lecture is aimed at giving a sufficient background on crystallography, as a reminder to ease the reading of the forthcoming chapters. It more precisely recalls the crystallographic restrictions on the space isometries, enumerates the point groups and the crystal lattices consistent with these, examines the structure of the space group, which gathers all the spatial invariances of a crystal, and describes a few dual notions. It next attempts to familiarize us with the representation analysis of physical states and excitations of crystals. 1. INTRODUCTION Crystallography covers a wide spectrum of investigations: i- it aspires to get an insight into crystallization phenomena and develops methods of crystal growths, which generally pertains to the physics of non linear irreversible processes; ii- it geometrically describes the natural shapes and the internal structures of the crystals, which is carried out most conveniently by borrowing mathematical tools from group theory; iii- it investigates the crystallized matter at the atomic scale by means of diffraction techniques using X-rays, electrons or neutrons, which are interpreted in the dual context of the reciprocal space and transposition therein of the crystal symmetries; iv- it analyzes the imperfections of the crystals, often directly visualized in scanning electron, tunneling or force microscopies, which in some instances find a meaning by handling unfamiliar concepts from homotopy theory; v- it aims at providing means for discerning the influences of the crystal structure on the physical properties of the materials, which requires to make use of mathematical methods from representation theory. -
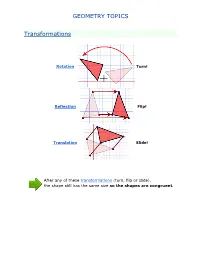
Geometry Topics
GEOMETRY TOPICS Transformations Rotation Turn! Reflection Flip! Translation Slide! After any of these transformations (turn, flip or slide), the shape still has the same size so the shapes are congruent. Rotations Rotation means turning around a center. The distance from the center to any point on the shape stays the same. The rotation has the same size as the original shape. Here a triangle is rotated around the point marked with a "+" Translations In Geometry, "Translation" simply means Moving. The translation has the same size of the original shape. To Translate a shape: Every point of the shape must move: the same distance in the same direction. Reflections A reflection is a flip over a line. In a Reflection every point is the same distance from a central line. The reflection has the same size as the original image. The central line is called the Mirror Line ... Mirror Lines can be in any direction. Reflection Symmetry Reflection Symmetry (sometimes called Line Symmetry or Mirror Symmetry) is easy to see, because one half is the reflection of the other half. Here my dog "Flame" has her face made perfectly symmetrical with a bit of photo magic. The white line down the center is the Line of Symmetry (also called the "Mirror Line") The reflection in this lake also has symmetry, but in this case: -The Line of Symmetry runs left-to-right (horizontally) -It is not perfect symmetry, because of the lake surface. Line of Symmetry The Line of Symmetry (also called the Mirror Line) can be in any direction. But there are four common directions, and they are named for the line they make on the standard XY graph.