Symmetry in Chemistry - Group Theory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In Order for the Figure to Map Onto Itself, the Line of Reflection Must Go Through the Center Point
3-5 Symmetry State whether the figure appears to have line symmetry. Write yes or no. If so, copy the figure, draw all lines of symmetry, and state their number. 1. SOLUTION: A figure has reflectional symmetry if the figure can be mapped onto itself by a reflection in a line. The figure has reflectional symmetry. In order for the figure to map onto itself, the line of reflection must go through the center point. Two lines of reflection go through the sides of the figure. Two lines of reflection go through the vertices of the figure. Thus, there are four possible lines that go through the center and are lines of reflections. Therefore, the figure has four lines of symmetry. eSolutionsANSWER: Manual - Powered by Cognero Page 1 yes; 4 2. SOLUTION: A figure has reflectional symmetry if the figure can be mapped onto itself by a reflection in a line. The given figure does not have reflectional symmetry. There is no way to fold or reflect it onto itself. ANSWER: no 3. SOLUTION: A figure has reflectional symmetry if the figure can be mapped onto itself by a reflection in a line. The given figure has reflectional symmetry. The figure has a vertical line of symmetry. It does not have a horizontal line of symmetry. The figure does not have a line of symmetry through the vertices. Thus, the figure has only one line of symmetry. ANSWER: yes; 1 State whether the figure has rotational symmetry. Write yes or no. If so, copy the figure, locate the center of symmetry, and state the order and magnitude of symmetry. -

Proofs with Perpendicular Lines
3.4 Proofs with Perpendicular Lines EEssentialssential QQuestionuestion What conjectures can you make about perpendicular lines? Writing Conjectures Work with a partner. Fold a piece of paper D in half twice. Label points on the two creases, as shown. a. Write a conjecture about AB— and CD — . Justify your conjecture. b. Write a conjecture about AO— and OB — . AOB Justify your conjecture. C Exploring a Segment Bisector Work with a partner. Fold and crease a piece A of paper, as shown. Label the ends of the crease as A and B. a. Fold the paper again so that point A coincides with point B. Crease the paper on that fold. b. Unfold the paper and examine the four angles formed by the two creases. What can you conclude about the four angles? B Writing a Conjecture CONSTRUCTING Work with a partner. VIABLE a. Draw AB — , as shown. A ARGUMENTS b. Draw an arc with center A on each To be prof cient in math, side of AB — . Using the same compass you need to make setting, draw an arc with center B conjectures and build a on each side of AB— . Label the C O D logical progression of intersections of the arcs C and D. statements to explore the c. Draw CD — . Label its intersection truth of your conjectures. — with AB as O. Write a conjecture B about the resulting diagram. Justify your conjecture. CCommunicateommunicate YourYour AnswerAnswer 4. What conjectures can you make about perpendicular lines? 5. In Exploration 3, f nd AO and OB when AB = 4 units. -

Molecular Symmetry
Molecular Symmetry Symmetry helps us understand molecular structure, some chemical properties, and characteristics of physical properties (spectroscopy) – used with group theory to predict vibrational spectra for the identification of molecular shape, and as a tool for understanding electronic structure and bonding. Symmetrical : implies the species possesses a number of indistinguishable configurations. 1 Group Theory : mathematical treatment of symmetry. symmetry operation – an operation performed on an object which leaves it in a configuration that is indistinguishable from, and superimposable on, the original configuration. symmetry elements – the points, lines, or planes to which a symmetry operation is carried out. Element Operation Symbol Identity Identity E Symmetry plane Reflection in the plane σ Inversion center Inversion of a point x,y,z to -x,-y,-z i Proper axis Rotation by (360/n)° Cn 1. Rotation by (360/n)° Improper axis S 2. Reflection in plane perpendicular to rotation axis n Proper axes of rotation (C n) Rotation with respect to a line (axis of rotation). •Cn is a rotation of (360/n)°. •C2 = 180° rotation, C 3 = 120° rotation, C 4 = 90° rotation, C 5 = 72° rotation, C 6 = 60° rotation… •Each rotation brings you to an indistinguishable state from the original. However, rotation by 90° about the same axis does not give back the identical molecule. XeF 4 is square planar. Therefore H 2O does NOT possess It has four different C 2 axes. a C 4 symmetry axis. A C 4 axis out of the page is called the principle axis because it has the largest n . By convention, the principle axis is in the z-direction 2 3 Reflection through a planes of symmetry (mirror plane) If reflection of all parts of a molecule through a plane produced an indistinguishable configuration, the symmetry element is called a mirror plane or plane of symmetry . -
![Arxiv:1910.10745V1 [Cond-Mat.Str-El] 23 Oct 2019 2.2 Symmetry-Protected Time Crystals](https://docslib.b-cdn.net/cover/4942/arxiv-1910-10745v1-cond-mat-str-el-23-oct-2019-2-2-symmetry-protected-time-crystals-304942.webp)
Arxiv:1910.10745V1 [Cond-Mat.Str-El] 23 Oct 2019 2.2 Symmetry-Protected Time Crystals
A Brief History of Time Crystals Vedika Khemania,b,∗, Roderich Moessnerc, S. L. Sondhid aDepartment of Physics, Harvard University, Cambridge, Massachusetts 02138, USA bDepartment of Physics, Stanford University, Stanford, California 94305, USA cMax-Planck-Institut f¨urPhysik komplexer Systeme, 01187 Dresden, Germany dDepartment of Physics, Princeton University, Princeton, New Jersey 08544, USA Abstract The idea of breaking time-translation symmetry has fascinated humanity at least since ancient proposals of the per- petuum mobile. Unlike the breaking of other symmetries, such as spatial translation in a crystal or spin rotation in a magnet, time translation symmetry breaking (TTSB) has been tantalisingly elusive. We review this history up to recent developments which have shown that discrete TTSB does takes place in periodically driven (Floquet) systems in the presence of many-body localization (MBL). Such Floquet time-crystals represent a new paradigm in quantum statistical mechanics — that of an intrinsically out-of-equilibrium many-body phase of matter with no equilibrium counterpart. We include a compendium of the necessary background on the statistical mechanics of phase structure in many- body systems, before specializing to a detailed discussion of the nature, and diagnostics, of TTSB. In particular, we provide precise definitions that formalize the notion of a time-crystal as a stable, macroscopic, conservative clock — explaining both the need for a many-body system in the infinite volume limit, and for a lack of net energy absorption or dissipation. Our discussion emphasizes that TTSB in a time-crystal is accompanied by the breaking of a spatial symmetry — so that time-crystals exhibit a novel form of spatiotemporal order. -

The Cubic Groups
The Cubic Groups Baccalaureate Thesis in Electrical Engineering Author: Supervisor: Sana Zunic Dr. Wolfgang Herfort 0627758 Vienna University of Technology May 13, 2010 Contents 1 Concepts from Algebra 4 1.1 Groups . 4 1.2 Subgroups . 4 1.3 Actions . 5 2 Concepts from Crystallography 6 2.1 Space Groups and their Classification . 6 2.2 Motions in R3 ............................. 8 2.3 Cubic Lattices . 9 2.4 Space Groups with a Cubic Lattice . 10 3 The Octahedral Symmetry Groups 11 3.1 The Elements of O and Oh ..................... 11 3.2 A Presentation of Oh ......................... 14 3.3 The Subgroups of Oh ......................... 14 2 Abstract After introducing basics from (mathematical) crystallography we turn to the description of the octahedral symmetry groups { the symmetry group(s) of a cube. Preface The intention of this account is to provide a description of the octahedral sym- metry groups { symmetry group(s) of the cube. We first give the basic idea (without proofs) of mathematical crystallography, namely that the 219 space groups correspond to the 7 crystal systems. After this we come to describing cubic lattices { such ones that are built from \cubic cells". Finally, among the cubic lattices, we discuss briefly the ones on which O and Oh act. After this we provide lists of the elements and the subgroups of Oh. A presentation of Oh in terms of generators and relations { using the Dynkin diagram B3 is also given. It is our hope that this account is accessible to both { the mathematician and the engineer. The picture on the title page reflects Ha¨uy'sidea of crystal structure [4]. -

Chapter 1 – Symmetry of Molecules – P. 1
Chapter 1 – Symmetry of Molecules – p. 1 - 1. Symmetry of Molecules 1.1 Symmetry Elements · Symmetry operation: Operation that transforms a molecule to an equivalent position and orientation, i.e. after the operation every point of the molecule is coincident with an equivalent point. · Symmetry element: Geometrical entity (line, plane or point) which respect to which one or more symmetry operations can be carried out. In molecules there are only four types of symmetry elements or operations: · Mirror planes: reflection with respect to plane; notation: s · Center of inversion: inversion of all atom positions with respect to inversion center, notation i · Proper axis: Rotation by 2p/n with respect to the axis, notation Cn · Improper axis: Rotation by 2p/n with respect to the axis, followed by reflection with respect to plane, perpendicular to axis, notation Sn Formally, this classification can be further simplified by expressing the inversion i as an improper rotation S2 and the reflection s as an improper rotation S1. Thus, the only symmetry elements in molecules are Cn and Sn. Important: Successive execution of two symmetry operation corresponds to another symmetry operation of the molecule. In order to make this statement a general rule, we require one more symmetry operation, the identity E. (1.1: Symmetry elements in CH4, successive execution of symmetry operations) 1.2. Systematic classification by symmetry groups According to their inherent symmetry elements, molecules can be classified systematically in so called symmetry groups. We use the so-called Schönfliess notation to name the groups, Chapter 1 – Symmetry of Molecules – p. 2 - which is the usual notation for molecules. -

TASI 2008 Lectures: Introduction to Supersymmetry And
TASI 2008 Lectures: Introduction to Supersymmetry and Supersymmetry Breaking Yuri Shirman Department of Physics and Astronomy University of California, Irvine, CA 92697. [email protected] Abstract These lectures, presented at TASI 08 school, provide an introduction to supersymmetry and supersymmetry breaking. We present basic formalism of supersymmetry, super- symmetric non-renormalization theorems, and summarize non-perturbative dynamics of supersymmetric QCD. We then turn to discussion of tree level, non-perturbative, and metastable supersymmetry breaking. We introduce Minimal Supersymmetric Standard Model and discuss soft parameters in the Lagrangian. Finally we discuss several mech- anisms for communicating the supersymmetry breaking between the hidden and visible sectors. arXiv:0907.0039v1 [hep-ph] 1 Jul 2009 Contents 1 Introduction 2 1.1 Motivation..................................... 2 1.2 Weylfermions................................... 4 1.3 Afirstlookatsupersymmetry . .. 5 2 Constructing supersymmetric Lagrangians 6 2.1 Wess-ZuminoModel ............................... 6 2.2 Superfieldformalism .............................. 8 2.3 VectorSuperfield ................................. 12 2.4 Supersymmetric U(1)gaugetheory ....................... 13 2.5 Non-abeliangaugetheory . .. 15 3 Non-renormalization theorems 16 3.1 R-symmetry.................................... 17 3.2 Superpotentialterms . .. .. .. 17 3.3 Gaugecouplingrenormalization . ..... 19 3.4 D-termrenormalization. ... 20 4 Non-perturbative dynamics in SUSY QCD 20 4.1 Affleck-Dine-Seiberg -

Observability and Symmetries of Linear Control Systems
S S symmetry Article Observability and Symmetries of Linear Control Systems Víctor Ayala 1,∗, Heriberto Román-Flores 1, María Torreblanca Todco 2 and Erika Zapana 2 1 Instituto de Alta Investigación, Universidad de Tarapacá, Casilla 7D, Arica 1000000, Chile; [email protected] 2 Departamento Académico de Matemáticas, Universidad, Nacional de San Agustín de Arequipa, Calle Santa Catalina, Nro. 117, Arequipa 04000, Peru; [email protected] (M.T.T.); [email protected] (E.Z.) * Correspondence: [email protected] Received: 7 April 2020; Accepted: 6 May 2020; Published: 4 June 2020 Abstract: The goal of this article is to compare the observability properties of the class of linear control systems in two different manifolds: on the Euclidean space Rn and, in a more general setup, on a connected Lie group G. For that, we establish well-known results. The symmetries involved in this theory allow characterizing the observability property on Euclidean spaces and the local observability property on Lie groups. Keywords: observability; symmetries; Euclidean spaces; Lie groups 1. Introduction For general facts about control theory, we suggest the references, [1–5], and for general mathematical issues, the references [6–8]. In the context of this article, a general control system S can be modeled by the data S = (M, D, h, N). Here, M is the manifold of the space states, D is a family of differential equations on M, or if you wish, a family of vector fields on the manifold, h : M ! N is a differentiable observation map, and N is a manifold that contains all the known information that you can see of the system through h. -

20. Geometry of the Circle (SC)
20. GEOMETRY OF THE CIRCLE PARTS OF THE CIRCLE Segments When we speak of a circle we may be referring to the plane figure itself or the boundary of the shape, called the circumference. In solving problems involving the circle, we must be familiar with several theorems. In order to understand these theorems, we review the names given to parts of a circle. Diameter and chord The region that is encompassed between an arc and a chord is called a segment. The region between the chord and the minor arc is called the minor segment. The region between the chord and the major arc is called the major segment. If the chord is a diameter, then both segments are equal and are called semi-circles. The straight line joining any two points on the circle is called a chord. Sectors A diameter is a chord that passes through the center of the circle. It is, therefore, the longest possible chord of a circle. In the diagram, O is the center of the circle, AB is a diameter and PQ is also a chord. Arcs The region that is enclosed by any two radii and an arc is called a sector. If the region is bounded by the two radii and a minor arc, then it is called the minor sector. www.faspassmaths.comIf the region is bounded by two radii and the major arc, it is called the major sector. An arc of a circle is the part of the circumference of the circle that is cut off by a chord. -
Geometry Course Outline
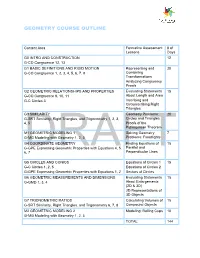
GEOMETRY COURSE OUTLINE Content Area Formative Assessment # of Lessons Days G0 INTRO AND CONSTRUCTION 12 G-CO Congruence 12, 13 G1 BASIC DEFINITIONS AND RIGID MOTION Representing and 20 G-CO Congruence 1, 2, 3, 4, 5, 6, 7, 8 Combining Transformations Analyzing Congruency Proofs G2 GEOMETRIC RELATIONSHIPS AND PROPERTIES Evaluating Statements 15 G-CO Congruence 9, 10, 11 About Length and Area G-C Circles 3 Inscribing and Circumscribing Right Triangles G3 SIMILARITY Geometry Problems: 20 G-SRT Similarity, Right Triangles, and Trigonometry 1, 2, 3, Circles and Triangles 4, 5 Proofs of the Pythagorean Theorem M1 GEOMETRIC MODELING 1 Solving Geometry 7 G-MG Modeling with Geometry 1, 2, 3 Problems: Floodlights G4 COORDINATE GEOMETRY Finding Equations of 15 G-GPE Expressing Geometric Properties with Equations 4, 5, Parallel and 6, 7 Perpendicular Lines G5 CIRCLES AND CONICS Equations of Circles 1 15 G-C Circles 1, 2, 5 Equations of Circles 2 G-GPE Expressing Geometric Properties with Equations 1, 2 Sectors of Circles G6 GEOMETRIC MEASUREMENTS AND DIMENSIONS Evaluating Statements 15 G-GMD 1, 3, 4 About Enlargements (2D & 3D) 2D Representations of 3D Objects G7 TRIONOMETRIC RATIOS Calculating Volumes of 15 G-SRT Similarity, Right Triangles, and Trigonometry 6, 7, 8 Compound Objects M2 GEOMETRIC MODELING 2 Modeling: Rolling Cups 10 G-MG Modeling with Geometry 1, 2, 3 TOTAL: 144 HIGH SCHOOL OVERVIEW Algebra 1 Geometry Algebra 2 A0 Introduction G0 Introduction and A0 Introduction Construction A1 Modeling With Functions G1 Basic Definitions and Rigid -

Pose Estimation for Objects with Rotational Symmetry
Pose Estimation for Objects with Rotational Symmetry Enric Corona, Kaustav Kundu, Sanja Fidler Abstract— Pose estimation is a widely explored problem, enabling many robotic tasks such as grasping and manipulation. In this paper, we tackle the problem of pose estimation for objects that exhibit rotational symmetry, which are common in man-made and industrial environments. In particular, our aim is to infer poses for objects not seen at training time, but for which their 3D CAD models are available at test time. Previous X 2 X 1 X 2 X 1 work has tackled this problem by learning to compare captured Y ∼ 2 Y ∼ 2 Y ∼ 2 Y ∼ 2 views of real objects with the rendered views of their 3D CAD Z ∼ 6 Z ∼ 1 Z ∼ Z ∼ 1 ∼ ∼ ∼ ∞ ∼ models, by embedding them in a joint latent space using neural Fig. 1. Many industrial objects such as various tools exhibit rotational networks. We show that sidestepping the issue of symmetry symmetries. In our work, we address pose estimation for such objects. in this scenario during training leads to poor performance at test time. We propose a model that reasons about rotational symmetry during training by having access to only a small set of that this is not a trivial task, as the rendered views may look symmetry-labeled objects, whereby exploiting a large collection very different from objects in real images, both because of of unlabeled CAD models. We demonstrate that our approach different background, lighting, and possible occlusion that significantly outperforms a naively trained neural network on arise in real scenes. -

Symmetry of Graphs. Circles
Symmetry of graphs. Circles Symmetry of graphs. Circles 1 / 10 Today we will be interested in reflection across the x-axis, reflection across the y-axis and reflection across the origin. Reflection across y reflection across x reflection across (0; 0) Sends (x,y) to (-x,y) Sends (x,y) to (x,-y) Sends (x,y) to (-x,-y) Examples with Symmetry What is Symmetry? Take some geometrical object. It is called symmetric if some geometric move preserves it Symmetry of graphs. Circles 2 / 10 Reflection across y reflection across x reflection across (0; 0) Sends (x,y) to (-x,y) Sends (x,y) to (x,-y) Sends (x,y) to (-x,-y) Examples with Symmetry What is Symmetry? Take some geometrical object. It is called symmetric if some geometric move preserves it Today we will be interested in reflection across the x-axis, reflection across the y-axis and reflection across the origin. Symmetry of graphs. Circles 2 / 10 Sends (x,y) to (-x,y) Sends (x,y) to (x,-y) Sends (x,y) to (-x,-y) Examples with Symmetry What is Symmetry? Take some geometrical object. It is called symmetric if some geometric move preserves it Today we will be interested in reflection across the x-axis, reflection across the y-axis and reflection across the origin. Reflection across y reflection across x reflection across (0; 0) Symmetry of graphs. Circles 2 / 10 Sends (x,y) to (-x,y) Sends (x,y) to (x,-y) Sends (x,y) to (-x,-y) Examples with Symmetry What is Symmetry? Take some geometrical object.