Updated Guidelines for the Diagnosis and Treatment of Acute Calculus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acute Onset Flank Pain-Suspicion of Stone Disease (Urolithiasis)
Date of origin: 1995 Last review date: 2015 American College of Radiology ® ACR Appropriateness Criteria Clinical Condition: Acute Onset Flank Pain—Suspicion of Stone Disease (Urolithiasis) Variant 1: Suspicion of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 8 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). US color Doppler kidneys and bladder 6 O retroperitoneal Radiography intravenous urography 4 ☢☢☢ MRI abdomen and pelvis without IV 4 MR urography. O contrast MRI abdomen and pelvis without and with 4 MR urography. O IV contrast This procedure can be performed with US X-ray abdomen and pelvis (KUB) 3 as an alternative to NCCT. ☢☢ CT abdomen and pelvis with IV contrast 2 ☢☢☢ *Relative Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate Radiation Level Variant 2: Recurrent symptoms of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 7 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated in an emergent setting for acute management to evaluate for hydronephrosis. For planning and US color Doppler kidneys and bladder 7 intervention, US is generally not adequate O retroperitoneal and CT is complementary as CT more accurately characterizes stone size and location. This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). -

Study of Calculus Pancreatitis
STUDY OF CALCULUS PANCREATITIS Dissertation Submitted for MS Degree (Branch I) General Surgery April 2011 The Tamilnadu Dr.M.G.R.Medical University Chennai – 600 032. MADURAI MEDICAL COLLEGE, MADURAI. CERTIFICATE This is to certify that this dissertation titled “STUDY OF CALCULUS PANCREATITIS” submitted by DR.P.K.PRABU to the faculty of General Surgery, The Tamilnadu Dr. M.G.R. Medical University, Chennai in partial fulfillment of the requirement for the award of MS degree Branch I General Surgery, is a bonafide research work carried out by him under our direct supervision and guidance from October 2008 to October 2010. DR. M.GOPINATH, M.S., Pro. A.SANKARAMAHALINGAM M.S, PROFESSOR AND HEAD, PROFESSOR, DEPARTMENT OF GENERAL SURGERY, DEPARTMENT OF GENERAL SURGERY, MADURAI MEDICAL COLLEGE, MADURAI MEDICAL COLLEGE, MADURAI. MADURAI. DECLARATION I, DR.P.K.PRABU solemnly declare that the dissertation titled “STUDY OF CALCULUS PANCREATITIS” has been prepared by me. This is submitted to The Tamilnadu Dr. M.G.R. Medical University, Chennai, in partial fulfillment of the regulations for the award of MS degree (Branch I) General Surgery. Place: Madurai DR. P.K.PRABU Date: ACKNOWLEDGEMENT At the very outset I would like to thank Dr.A.EDWIN JOE M.D.,(FM) the Dean Madurai Medical College and Dr.S.M.SIVAKUMAR M.S., (General Surgery) Medical Superintendent, Government Rajaji Hospital, Madurai for permitting me to carryout this study in this Hospital. I wish to express my sincere thanks to my Head of the Department of Surgery Prof.Dr.M.GOPINATH M.S., and Prof.Dr.MUTHUKRISHNAN M.Ch., Head of the Department of Surgical Gastroenterology for his unstinted encouragement and valuable guidance during this study. -

Postoperative Intrahepatic Calculus: the Role of Extracorporeal Shockwave Lithotripsy
Published online: 2021-04-10 Case Report Postoperative Intrahepatic Calculus: The Role of Extracorporeal Shockwave Lithotripsy Abstract Asad Irfanullah, Bile duct stones are a known complication after a Roux-en-Y hepaticojejunostomy. Different minimally Kamran Masood, invasive stone extraction techniques, including endoscopic retrograde cholangiopancreatography with Yousuf Memon, basket removal or the use of a choledocoscope through a mature T-tube tract, can be used. However, in some cases, they are unsuccessful due to complicated postsurgical anatomy or technical difficulty. Zakariya Irfanullah In this report, we present a case where extracorporeal shockwave lithotripsy was used in conjunction Department of Radiology, Indus with standard interventional techniques to treat bile duct stones. Hospital, Karachi, Pakistan Keywords: Biliary tract calculus, extracorporeal shockwave lithotripsy, post‑Roux‑en‑y hepaticojejunostomy Introduction medical history was significant for an open cholecystectomy complicated by Bile duct stones and anastomotic strictures iatrogenic injury to the common bile duct are known complications of Roux-en-y and subsequent creation of a Roux-en-y hepaticojejunostomy. Due to the postsurgical hepaticojejunostomy (REHJ). A magnetic anatomy, conventional endoscopic resonance cholangiopancreatography was retrograde cholangiopancreatography performed which demonstrated a significant (ERCP) techniques are often not possible. intrahepatic biliary dilatation with the In this specific case, we treated a large bile formation -
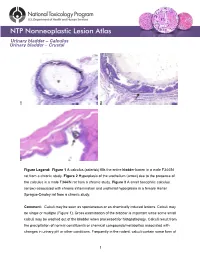
Urinary Bladder – Calculus Urinary Bladder – Crystal
Urinary bladder – Calculus Urinary bladder – Crystal Figure Legend: Figure 1 A calculus (asterisk) fills the entire bladder lumen in a male F344/N rat from a chronic study. Figure 2 Hyperplasia of the urothelium (arrow) due to the presence of the calculus in a male F344/N rat from a chronic study. Figure 3 A small basophilic calculus (arrow) associated with chronic inflammation and urothelial hyperplasia in a female Harlan Sprague-Dawley rat from a chronic study. Comment: Calculi may be seen as spontaneous or as chemically induced lesions. Calculi may be single or multiple (Figure 1). Gross examination of the bladder is important since some small calculi may be washed out of the bladder when processed for histopathology. Calculi result from the precipitation of normal constituents or chemical compounds/metabolites associated with changes in urinary pH or other conditions. Frequently in the rodent, calculi contain some form of 1 Urinary bladder – Calculus Urinary bladder – Crystal calcium or mineral complex. Calculi often result in necrosis, ulceration, inflammation, and hyperplasia of the urothelium (Figure 2 and Figure 3). They are often the cause of bladder obstruction. In addition, bladder neoplasia may result from the presence of calculi. The presence of crystals and the subsequent appearance of calculi are often associated. Strain differences in the presence of crystals have been reported. Crystals, like calculi, tend to be washed out during histologic processing. Recommendation: Calculi and crystals should be diagnosed but should not be graded. Calculi are usually associated with secondary lesions, such as hemorrhage and inflammation. The pathologist should use his or her judgment in deciding whether or not these secondary lesions are prominent enough to warrant a separate diagnosis. -

Obstruction of the Urinary Tract 2567
Chapter 540 ◆ Obstruction of the Urinary Tract 2567 Table 540-1 Types and Causes of Urinary Tract Obstruction LOCATION CAUSE Infundibula Congenital Calculi Inflammatory (tuberculosis) Traumatic Postsurgical Neoplastic Renal pelvis Congenital (infundibulopelvic stenosis) Inflammatory (tuberculosis) Calculi Neoplasia (Wilms tumor, neuroblastoma) Ureteropelvic junction Congenital stenosis Chapter 540 Calculi Neoplasia Inflammatory Obstruction of the Postsurgical Traumatic Ureter Congenital obstructive megaureter Urinary Tract Midureteral structure Jack S. Elder Ureteral ectopia Ureterocele Retrocaval ureter Ureteral fibroepithelial polyps Most childhood obstructive lesions are congenital, although urinary Ureteral valves tract obstruction can be caused by trauma, neoplasia, calculi, inflam- Calculi matory processes, or surgical procedures. Obstructive lesions occur at Postsurgical any level from the urethral meatus to the calyceal infundibula (Table Extrinsic compression 540-1). The pathophysiologic effects of obstruction depend on its level, Neoplasia (neuroblastoma, lymphoma, and other retroperitoneal or pelvic the extent of involvement, the child’s age at onset, and whether it is tumors) acute or chronic. Inflammatory (Crohn disease, chronic granulomatous disease) ETIOLOGY Hematoma, urinoma Ureteral obstruction occurring early in fetal life results in renal dys- Lymphocele plasia, ranging from multicystic kidney, which is associated with ure- Retroperitoneal fibrosis teral or pelvic atresia (see Fig. 537-2 in Chapter 537), to various -

Upper Gastrointestinal Endoscopy Policy Number: PG0449 ADVANTAGE | ELITE | HMO Last Review: 11/28/2018
Upper Gastrointestinal Endoscopy Policy Number: PG0449 ADVANTAGE | ELITE | HMO Last Review: 11/28/2018 INDIVIDUAL MARKETPLACE | PROMEDICA MEDICARE PLAN | PPO GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated by each individual policyholder contract. Paramount applies coding edits to all medical claims through coding logic software to evaluate the accuracy and adherence to accepted national standards. This guideline is solely for explaining correct procedure reporting and does not imply coverage and reimbursement. SCOPE X Professional _ Facility DESCRIPTION Upper gastrointestinal (GI) endoscopy, or esophagogastroduodenoscopy (EGD) is usually performed to evaluate symptoms of persistent upper abdominal pain, nausea, vomiting, and difficulty swallowing or bleeding from the upper GI tract. EGD is more accurate than x-ray films for detecting inflammation, ulcers, or tumors of the esophagus, stomach and duodenum and can detect early cancer, as well as distinguish between benign and malignant conditions when biopsies of suspicious areas are obtained. Esophagogastroduodenoscopy (EGD) uses a flexible fiber-optic scope with a light and camera to examine the upper part of the GI system. The scope is inserted through the mouth into the upper GI tract allowing for direct visualization of the esophagus, stomach, and duodenum through the camera. This document does not address upper gastrointestinal (GI) endoscopy in children, wireless capsule endoscopy, virtual endoscopy or in vivo analysis of gastrointestinal lesions via endoscopy. POLICY Upper gastrointestinal endoscopy does not require prior authorization. Appropriate ICD-10 diagnosis code (as listed below) required for coverage. COVERAGE CRITERIA HMO, PPO, Individual Marketplace, Elite/ProMedica Medicare Plan, Advantage These procedures for adults aged 18 years or older can only be allowed if abnormal signs or symptoms or known disease are present. -

Gallbladder Function Predicts Subsequent Biliary Complications In
Tsai et al. BMC Gastroenterology (2018) 18:32 https://doi.org/10.1186/s12876-018-0762-6 RESEARCH ARTICLE Open Access Gallbladder function predicts subsequent biliary complications in patients with common bile duct stones after endoscopic treatment? Tzung-Jiun Tsai1 , Hoi-Hung Chan1,2,4,5,6*, Kwok-Hung Lai1,2, Chih-An Shih1, Sung-Shuo Kao1,2, Wei-Chih Sun1, E-Ming Wang1, Wei-Lun Tsai1,2, Kung-Hung Lin1, Hsien-Chung Yu1, Wen-Chi Chen1,2, Huay-Min Wang1, Feng-Woei Tsay1, Huey-Shyan Lin3, Jin-Shiung Cheng1 and Ping-I Hsu1,2 Abstracts Background: In patients with common bile duct stones (CBDS) and intact gallbladder, further management for the gallbladder after the CBDS clearance is still controversial. The relationship between gallbladder motility and the biliary complications were seldom discussed. Our study is to predict the subsequent biliary complications by gallbladder function test using fatty meal sonography (FMS) in patients with CBDS who had been treated by endoscopic retrograde cholangiopancreatography (ERCP). Methods: Patients with an intact gallbladder and CBDS after endoscopic clearance of bile duct were enrolled. Patients received a fatty meal sonography after liver function returned to normal. The fasting volume, residual volume, and gallbladder ejection fraction (GBEF) in FMS were measured. Relationships of patients’ characteristics, gallbladder function and recurrent biliary complication were analyzed. Results: From 2011 to 2014, 118 patients were enrolled; 86 patients had calculus gallbladders, and 32 patients had acalculous gallbladders. After a mean follow- up of 33 months, 23 patients had recurrent biliary complications. Among 86 patients with calculus gallbladder, 15 patients had spontaneous clearance of gallbladder stones; 14 patients received cholecystectomy due to acute cholecystitis or recurrent colic pain with smooth postoperative courses. -

Nephrolithiasis, Urinary Stones, Kidney, Calciuria, Oxaluria
KIDNEY STONES KEYWORDS: Nephrolithiasis, urinary stones, kidney, calciuria, oxaluria. LEARNING OBJECTIVES: At the end of medical school, the medical student will be able to… List risk factors for the most common types of kidney stones Contrast differences between the clinical presentation of acute renal colic versus an acute abdomen Name 4 kidney stone chemical compositions Describe the best imaging study to diagnose kidney or ureteral stones Describe 3 types of medications effective for relief of renal colic pain List 3 clinical situations that warrant urgent decompression of a ureteral stone List 2 types of medications that may help medical expulsion therapy of a distal ureteral stone Describe two medical prophylaxis options for hypercalciuria List 2 common surgical techniques to manage a renal stone and a ureteral stone that fails to pass with observation INTRODUCTION Urinary stone prevalence is estimated at 3% in all individuals, and it affects up to 12% of the population during their lifetime. Urinary stone recurrence rates approach 50% at 10 years and white males have the highest incidence in the U.S. There is traditionally a high incidence of urinary stones in the southeastern and central southern United States, termed the “Stone Belt”, which probably reflects hot weather and dehydration that occur in these areas. Prior to the development of modern urologic techniques for treatment, mortality from untreated staghorn calculi was 27%. Currently mortality from stone disease is rare, although there is still a significant rate (28%) of renal deterioration with certain stone types. PATHOPHYSIOLOGY Urinary calculi may have various compositions which include, in order of decreasing frequency: calcium oxalate (monohydrate or dihydrate), uric acid, struvite (magnesium ammonium phosphate), calcium phosphate, and cystine. -

Antibiotics in the Treatment of Biliary Infection
Gut: first published as 10.1136/gut.25.9.988 on 1 September 1984. Downloaded from Gut, 1984, 25, 988-998 Progress report Antibiotics in the treatment of biliary infection Bile is difficult to obtain for culture in man. Because of this, antibiotic treatment of biliary infection is often directed towards biliary organisms which are suspected, rather than proven. To complicate effective treatment further, infection may be asymptomatic, presenting with septic complications following a surgical or radiological procedure. Despite knowledge of the pattern of infection in biliary disease, the microorganisms involved, and the spectrum and pharmacokinetics of potentially effective antibiotics, bacteria are often difficult to eradicate from the bile and biliary surgery has a high incidence of septic complications. There have been few reviews of this subject, particularly incorporating recent data, and a reassessment is necessary. This paper describes the clinical and bacteriological aspects of biliary infection, and the excretion and efficacy of specific antibiotics in bile. Based on the information available a therapeutic strategy is proposed. Biliary infection http://gut.bmj.com/ DEFINITION Ideally biliary tract infection should be defined by the organism count in bile. Keighley et al' reported finding at least 105 organisms per ml in over 90% of positive peroperative bile cultures, and this count could be taken as diagnostic. Such a definition is, however, impracticable because bile is difficult to collect. Although biliary sepsis is diagnosed clinically when there are systemic signs of infection combined with features of biliary tract on September 27, 2021 by guest. Protected copyright. disease, organisms may be cultured from bile in the absence of symptoms. -

Calculous Disease of the Vermiform Appendix
Gut: first published as 10.1136/gut.7.6.583 on 1 December 1966. Downloaded from Gut, 1966, 7, 583 Calculous disease of the vermiform appendix G. B. FORBES AND R. W. LLOYD-DAVIES From the Kent and Canterbury Hospital, Canterbury EDITORIAL SYNOPSIS Calculi were found in 29 out of 1,800 vermiform appendices removed at operation. The high incidence of acute complications associated with a calculus indicates the need to remove the appendix when one is found incidentally on radiological examination of the abdomen. The formation of true calculi within the lumen of the arbitrary and indeed the clinical effects and compli- vermiform appendix is a comparatively rare event, cations of both types of calcified object, whether but the condition is of clinical importance because of described as faecolith or calculus, are likely to be the frequency with which such calculi give rise to very similar. When estimating the incidence of serious complications. The main clinico-pathological calculous disease, however, it is necessary to have features of calculous disease of the appendix, based more precise criteria for differentiating the various on the examination of 2,000 appendix specimens, formed faecal objects encountered in appendicec- are presented in this report with the following tomy specimens. In the present study all such objects objectives in view: to clarify the terminology of the were grouped on the basis of their physical charac- various formed faecal objects which are found in the ters, using the following key as an aid to identifica- lumen of the appendix; to assess the incidence of tion: calculous disease and its complications; to draw the attention of surgeons, radiologists, and pathologists 1 FAECAL PELLET This is a formed, firm, but not to a disease which deserves wider recognition. -

GB Perforation with Contained Biloma a Rare Complication of Calculus Cholecystitis
Case Report Annals of Surgical Case Reports Published: 11 Sep, 2020 GB Perforation with Contained Biloma a Rare Complication of Calculus Cholecystitis Krishnanand1, Sakshi Goyal1*, Amit Tiwari1, Pranav Kumar Dave2 1Department of General Surgery, L.N. Medical College and J.K.Hospital, Bhopal 2Department of Radiodiagnosis, L.N. Medical College and J.K.Hospital, Bhopal Abstract Gall Bladder Perforation (GBP) is a rare and potentially life threatening complication of acute cholecystitis. The main cause of GBP is cholecystitis with or without cholelithiasis and is often associated with high morbidity and mortality. It is subdivided into three categories whereas the development of biloma is extremely rare. We report an interesting case of Gall bladder perforation after acute calculus cholecystitis causing sub hepatic biloma formation who presented in emergency with pain in right upper abdomen and fever. Keywords: Acute cholecystitis; Gallbladder perforation; Biloma Introduction Gall bladder perforation (GBP) is an uncommon complication of acute cholecystitis with an incidence rate of 0.8 4.8% and a mortality rate of 9.5% to 16% [1,2]. GBP due to a calculus cholecystitis is more common than calculus cholecystitis. It has male preponderance of above 60 years with systematic diseases [2,3]. The main cause of gall bladder perforation is cholecystitis with or without cholelithiasis [4]. Usually patients with GBP present with localized or diffuse peritonitis causing dilemma in early diagnosis. Radiological evaluations serve a vital role in early identification and appropriate intervention. The proposed mechanism of GBP is persistent inflammation and increased intra cholecystic pressure due to impacted stone leading to ischemia, necrosis, and perforation. -

The Evolution of Surgical Treatment of Urolithiasis: from Lithotomy to Combined Intrarenal Technologies
Urology & Nephrology Open Access Journal Review Article Open Access The evolution of surgical treatment of urolithiasis: from lithotomy to combined intrarenal technologies Summary Volume 9 Issue 2 - 2021 Urolithiasis (hereinafter ICD) has a long thousand-year history, worldwide spread, frequent Rogachikov VV, Bogorad IV, Kudryashov AV, recurrence and occupies a leading place in the structure of surgical diseases of the urinary system. The development of urology as a clinical discipline is thorny and is associated Ignatiev DN Department of urology, Russia with the stages of origin and formation as a science, oblivion and revival - as a modern field of surgery. The progress of technical thought, achievements of fundamental science, Correspondence: Rogachikov VV, Department of urology, naturally led to the formation of urology and, in particular, surgery of urolithiasis on the Russia, Email basis of new concepts of operative capabilities, preoperative diagnostics, new methods of physical impact on the structure of the calculus. The creation and improvement of new low- Received: February 26, 2021 | Published: March 15, 2021 traumatic techniques, their active and widespread introduction into practice, contributed to the displacement of open traumatic interventions used for centuries, and made it possible to successfully remove stones from the urinary tract with minimal complications. The work is devoted to the historical review of the formation of urolithiasis surgery, comparative characteristics of alternative minimally invasive methods of treatment of KSD. The scientific work reveals the modern achievements of percutaneous surgery, the history of the development of technical improvements and the possibilities of retrograde fiber- optic intrarenal technologies. The promising directions of development of antegrade and retrograde surgery of nephrolithiasis in the present and near future have been determined.