Upper Gastrointestinal Endoscopy Policy Number: PG0449 ADVANTAGE | ELITE | HMO Last Review: 11/28/2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Colorectal Cancer Screening
CLINICAL MEDICAL POLICY Policy Name: Colorectal Cancer Screening Policy Number: MP-059-MD-PA Responsible Department(s): Medical Management Provider Notice Date: 03/19/2021 Issue Date: 03/19/2021 Effective Date: 04/19/2021 Next Annual Review: 02/2022 Revision Date: 02/17/2021 Products: Gateway Health℠ Medicaid Application: All participating hospitals and providers Page Number(s): 1 of 10 DISCLAIMER Gateway Health℠ (Gateway) medical policy is intended to serve only as a general reference resource regarding coverage for the services described. This policy does not constitute medical advice and is not intended to govern or otherwise influence medical decisions. POLICY STATEMENT Gateway Health℠ may provide coverage under the medical-surgical benefits of the Company’s Medicaid products for medically necessary colorectal cancer screening procedures. This policy is designed to address medical necessity guidelines that are appropriate for the majority of individuals with a particular disease, illness or condition. Each person’s unique clinical circumstances warrant individual consideration, based upon review of applicable medical records. (Current applicable Pennsylvania HealthChoices Agreement Section V. Program Requirements, B. Prior Authorization of Services, 1. General Prior Authorization Requirements.) Policy No. MP-059-MD-PA Page 1 of 10 DEFINITIONS Average-Risk Population – Patient population defined as having no personal history of adenomatous polyps, colorectal cancer, or inflammatory bowel disease (Crohn’s disease and Ulcerative Colitis); no family history of colorectal cancer or adenomatous polyps, familial adenomatous polyposis, or hereditary nonpolyposis colorectal cancer. High-Risk Population – Patient population defined as having a first-degree relative (sibling, parent, or child) who has had colorectal cancer or adenomatous polyps; OR family history of familial adenomatous polyposis; OR family history of hereditary non-polyposis colorectal cancer; OR family history of MYH- associated polyposis in siblings; OR diagnosis of Cowden syndrome. -

Acute Onset Flank Pain-Suspicion of Stone Disease (Urolithiasis)
Date of origin: 1995 Last review date: 2015 American College of Radiology ® ACR Appropriateness Criteria Clinical Condition: Acute Onset Flank Pain—Suspicion of Stone Disease (Urolithiasis) Variant 1: Suspicion of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 8 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). US color Doppler kidneys and bladder 6 O retroperitoneal Radiography intravenous urography 4 ☢☢☢ MRI abdomen and pelvis without IV 4 MR urography. O contrast MRI abdomen and pelvis without and with 4 MR urography. O IV contrast This procedure can be performed with US X-ray abdomen and pelvis (KUB) 3 as an alternative to NCCT. ☢☢ CT abdomen and pelvis with IV contrast 2 ☢☢☢ *Relative Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate Radiation Level Variant 2: Recurrent symptoms of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 7 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated in an emergent setting for acute management to evaluate for hydronephrosis. For planning and US color Doppler kidneys and bladder 7 intervention, US is generally not adequate O retroperitoneal and CT is complementary as CT more accurately characterizes stone size and location. This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). -

Geisinger Lewistown Hospital Published: March 25, 2019
Geisinger Lewistown Hospital Published: March 25, 2019 DESCRIPTION CHARGE Fine needle aspiration; without imaging guidance $ 607.00 Fine needle aspiration; without imaging guidance $ 286.00 Fine needle aspiration; with imaging guidance $ 2,218.00 Fine needle aspiration; with imaging guidance $ 1,691.00 Placement of soft tissue localization device(s) (eg, clip, metallic pellet, wire/needle, radioactive seeds), percutaneous, including imaging guidance; first lesion $ 1,979.00 Placement of soft tissue localization device(s) (eg, clip, metallic pellet, wire/needle, radioactive seeds), percutaneous, including imaging guidance; each $ 1,385.00 additional lesion (List separately in addition to code for primary procedure) Incision and drainage of abscess (eg, carbuncle, suppurative hidradenitis, cutaneous or subcutaneous abscess, cyst, furuncle, or paronychia); simple or single $ 657.00 Incision and drainage of abscess (eg, carbuncle, suppurative hidradenitis, cutaneous or subcutaneous abscess, cyst, furuncle, or paronychia); complicated or $ 986.00 multiple Incision and drainage of pilonidal cyst; simple $ 657.00 Incision and drainage of pilonidal cyst; complicated $ 3,726.00 Incision and removal of foreign body, subcutaneous tissues; simple $ 1,694.00 Incision and removal of foreign body, subcutaneous tissues; complicated $ 4,710.00 Incision and drainage of hematoma, seroma or fluid collection $ 3,470.00 Puncture aspiration of abscess, hematoma, bulla, or cyst $ 1,272.00 Puncture aspiration of abscess, hematoma, bulla, or cyst $ 657.00 Incision -

Confocal Laser Microscopy in Neurosurgery: State of the Art of Actual Clinical Applications
Journal of Clinical Medicine Review Confocal Laser Microscopy in Neurosurgery: State of the Art of Actual Clinical Applications Francesco Restelli 1, Bianca Pollo 2 , Ignazio Gaspare Vetrano 1 , Samuele Cabras 1, Morgan Broggi 1 , Marco Schiariti 1, Jacopo Falco 1, Camilla de Laurentis 1, Gabriella Raccuia 1, Paolo Ferroli 1 and Francesco Acerbi 1,* 1 Department of Neurosurgery, Fondazione IRCCS Istituto Neurologico Carlo Besta, 20133 Milan, Italy; [email protected] (F.R.); [email protected] (I.G.V.); [email protected] (S.C.); [email protected] (M.B.); [email protected] (M.S.); [email protected] (J.F.); [email protected] (C.d.L.); [email protected] (G.R.); [email protected] (P.F.) 2 Neuropathology Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, 20133 Milan, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-022-3932-309 Abstract: Achievement of complete resections is of utmost importance in brain tumor surgery, due to the established correlation among extent of resection and postoperative survival. Various tools have recently been included in current clinical practice aiming to more complete resections, such as neuronavigation and fluorescent-aided techniques, histopathological analysis still remains the gold-standard for diagnosis, with frozen section as the most used, rapid and precise intraoperative histopathological method that permits an intraoperative differential diagnosis. Unfortunately, due Citation: Restelli, F.; Pollo, B.; to the various limitations linked to this technique, it is still unsatisfactorily for obtaining real-time Vetrano, I.G.; Cabras, S.; Broggi, M.; intraoperative diagnosis. -

Study of Calculus Pancreatitis
STUDY OF CALCULUS PANCREATITIS Dissertation Submitted for MS Degree (Branch I) General Surgery April 2011 The Tamilnadu Dr.M.G.R.Medical University Chennai – 600 032. MADURAI MEDICAL COLLEGE, MADURAI. CERTIFICATE This is to certify that this dissertation titled “STUDY OF CALCULUS PANCREATITIS” submitted by DR.P.K.PRABU to the faculty of General Surgery, The Tamilnadu Dr. M.G.R. Medical University, Chennai in partial fulfillment of the requirement for the award of MS degree Branch I General Surgery, is a bonafide research work carried out by him under our direct supervision and guidance from October 2008 to October 2010. DR. M.GOPINATH, M.S., Pro. A.SANKARAMAHALINGAM M.S, PROFESSOR AND HEAD, PROFESSOR, DEPARTMENT OF GENERAL SURGERY, DEPARTMENT OF GENERAL SURGERY, MADURAI MEDICAL COLLEGE, MADURAI MEDICAL COLLEGE, MADURAI. MADURAI. DECLARATION I, DR.P.K.PRABU solemnly declare that the dissertation titled “STUDY OF CALCULUS PANCREATITIS” has been prepared by me. This is submitted to The Tamilnadu Dr. M.G.R. Medical University, Chennai, in partial fulfillment of the regulations for the award of MS degree (Branch I) General Surgery. Place: Madurai DR. P.K.PRABU Date: ACKNOWLEDGEMENT At the very outset I would like to thank Dr.A.EDWIN JOE M.D.,(FM) the Dean Madurai Medical College and Dr.S.M.SIVAKUMAR M.S., (General Surgery) Medical Superintendent, Government Rajaji Hospital, Madurai for permitting me to carryout this study in this Hospital. I wish to express my sincere thanks to my Head of the Department of Surgery Prof.Dr.M.GOPINATH M.S., and Prof.Dr.MUTHUKRISHNAN M.Ch., Head of the Department of Surgical Gastroenterology for his unstinted encouragement and valuable guidance during this study. -

EGD) Is a Common Endoscopic Procedure Done for Suspected and Proven Lesions of the Upper Gastrointestinal Tract
CLINICAL MEDICAL POLICY Upper Gastrointestinal Endoscopy and Visualization Policy Name: (L34434) Policy Number: MP-073-MC-NC Responsible Department(s): Medical Management Provider Notice Date: 07/01/2018 Issue Date: 08/01/2018 Effective Date: 08/01/2018 Annual Approval Date: 05/16/2019 Revision Date: N/A Products: North Carolina Medicare Assured All participating and nonparticipating hospitals and Application: providers Page Number(s): 1 of 22 DISCLAIMER Gateway Health℠ (Gateway) medical policy is intended to serve only as a general reference resource regarding coverage for the services described. This policy does not constitute medical advice and is not intended to govern or otherwise influence medical decisions. POLICY STATEMENT Gateway Health℠ may provide coverage under the medical-surgical benefits of the Company’s Medicare products for medically necessary Upper Gastrointestinal Endoscopy and Visualization. This policy is designed to address medical necessity guidelines that are appropriate for the majority of individuals with a particular disease, illness or condition. Each person’s unique clinical circumstances warrant individual consideration, based upon review of applicable medical records. Policy No. MP-037-MC-NC Page 1 of 22 PROCEDURES CMS NATIONAL COVERAGE POLICY Title XVIII of the Social Security Act, §1862(a)(1)(A) allows coverage and payment for only those services that are considered to be reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member. Title XVIII of the Social Security Act §1833(e) prohibits Medicare payment for any claim which lacks the necessary information to process the claim. Title XVIII of the Social Security Act §1862(a)(7) excludes routine physical examinations. -

Postoperative Intrahepatic Calculus: the Role of Extracorporeal Shockwave Lithotripsy
Published online: 2021-04-10 Case Report Postoperative Intrahepatic Calculus: The Role of Extracorporeal Shockwave Lithotripsy Abstract Asad Irfanullah, Bile duct stones are a known complication after a Roux-en-Y hepaticojejunostomy. Different minimally Kamran Masood, invasive stone extraction techniques, including endoscopic retrograde cholangiopancreatography with Yousuf Memon, basket removal or the use of a choledocoscope through a mature T-tube tract, can be used. However, in some cases, they are unsuccessful due to complicated postsurgical anatomy or technical difficulty. Zakariya Irfanullah In this report, we present a case where extracorporeal shockwave lithotripsy was used in conjunction Department of Radiology, Indus with standard interventional techniques to treat bile duct stones. Hospital, Karachi, Pakistan Keywords: Biliary tract calculus, extracorporeal shockwave lithotripsy, post‑Roux‑en‑y hepaticojejunostomy Introduction medical history was significant for an open cholecystectomy complicated by Bile duct stones and anastomotic strictures iatrogenic injury to the common bile duct are known complications of Roux-en-y and subsequent creation of a Roux-en-y hepaticojejunostomy. Due to the postsurgical hepaticojejunostomy (REHJ). A magnetic anatomy, conventional endoscopic resonance cholangiopancreatography was retrograde cholangiopancreatography performed which demonstrated a significant (ERCP) techniques are often not possible. intrahepatic biliary dilatation with the In this specific case, we treated a large bile formation -

Diagnostic and Therapeutic Endoscopy of Biliary Diseases
DISEASES OF THE BILIARY TRACT, SERIES #6 Rad Agrawal, M.D., Series Editor Diagnostic and Therapeutic Endoscopy of Biliary Diseases by Yamini Subbiah, Shyam Thakkar, Elie Aoun The therapeutic approach to biliary diseases has undergone a paradigm shift over the past decade toward minimally invasive endoscopic interventions. This paper reviews the advances and different diagnostic and therapeutic endoscopic approaches to common biliary diseases including choledocholithiasis, benign and malignant biliary strictures and bile leaks. INTRODUCTION endoscopist to differentiate between benign and malig- ith the introduction of innovative endoscopic nant features, thus guiding decision making in real time. implements and options allowing for unprece- Wdented access to the biliary tree, the therapeutic COMMON BILE DUCT STONES approach to biliary diseases has undergone a significant Over 98% of biliary disorders are linked to gallstones. paradigm shift over the past decade toward minimally Stones are found in the common bile duct (CBD) in up invasive endoscopic interventions. The days where bil- to 18% of patients with symptomatic cholelithiasis (1). iary diseases were exclusively managed surgically are The vast majority of gallstones are cholesterol-rich, long gone, and much has changed since the first form in the gallbladder and gain access to the CBD via reported biliary sphincterotomies in 1974. The recent the cystic duct. De novo CBD stone formation is also developments in peroral cholangioscopy and new well described and is more common in patients of modalities of anchoring high resolution nasogastric Asian descent. These primary duct stones typically scopes in the bile duct offer the opportunity of direct have a higher bilirubin and a lower cholesterol content visualization of the bile duct lumen, which allows for and biliary stasis; further, bacterial infections have not only better identification of the underlying disease been implicated in their pathogenesis (2,3). -

Confocal Laser Endomicroscopy Platform Large Addressable Market: Early Cancer Diagnosis in GI, Urology, Interventional 2 Pulmonology, Others
Mauna Kea Technologies Corporate Presentation - November 2018 Disclaimer • This document has been prepared by Mauna Kea Technologies (the "Company") and is provided for information purposes only. • The information and opinions contained in this document speak only as of the date of this document and may be updated, supplemented, revised, verified or amended, and such information may be subject to significant changes. Mauna Kea Technologies is not under any obligation to update the information contained herein and any opinion expressed in this document is subject to change without prior notice. • The information contained in this document has not been independently verified. No representation, warranty or undertaking, express or implied, is made as to the accuracy, completeness or appropriateness of the information and opinions contained in this document. The Company, its subsidiary, its advisors and representatives accept no responsibility for and shall not be held liable for any loss or damage that may arise from the use of this document or the information or opinions contained herein. • This document contains information on the Company’s markets and competitive position, and more specifically, on the size of its markets. This information has been drawn from various sources or from the Company’s own estimates. Investors should not base their investment decision on this information. • This document contains certain forward-looking statements. These statements are not guarantees of the Company's future performance. These forward- looking statements relate to the Company's future prospects, developments and marketing strategy and are based on analyses of earnings forecasts and estimates of amounts not yet determinable. Forward-looking statements are subject to a variety of risks and uncertainties as they relate to future events and are dependent on circumstances that may or may not materialize in the future. -
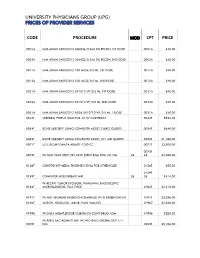
Code Procedure Cpt Price University Physicians Group
UNIVERSITY PHYSICIANS GROUP (UPG) PRICES OF PROVIDER SERVICES CODE PROCEDURE MOD CPT PRICE 0001A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 1ST DOSE 0001A $40.00 0002A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 2ND DOSE 0002A $40.00 0011A IMM ADMN SARSCOV2 100 MCG/0.5 ML 1ST DOSE 0011A $40.00 0012A IMM ADMN SARSCOV2 100 MCG/0.5 ML 2ND DOSE 0012A $40.00 0021A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 1ST DOSE 0021A $40.00 0022A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 2ND DOSE 0022A $40.00 0031A IMM ADMN SARSCOV2 AD26 5X10^10 VP/0.5 ML 1 DOSE 0031A $40.00 0042T CEREBRAL PERFUS ANALYSIS, CT W/CONTRAST 0042T $954.00 0054T BONE SURGERY USING COMPUTER ASSIST, FLURO GUIDED 0054T $640.00 0055T BONE SURGERY USING COMPUTER ASSIST, CT/ MRI GUIDED 0055T $1,188.00 0071T U/S LEIOMYOMATA ABLATE <200 CC 0071T $2,500.00 0075T 0075T PR TCAT PLMT XTRC VRT CRTD STENT RS&I PRQ 1ST VSL 26 26 $2,208.00 0126T CAROTID INT-MEDIA THICKNESS EVAL FOR ATHERSCLER 0126T $55.00 0159T 0159T COMPUTER AIDED BREAST MRI 26 26 $314.00 PR RECTAL TUMOR EXCISION, TRANSANAL ENDOSCOPIC 0184T MICROSURGICAL, FULL THICK 0184T $2,315.00 0191T PR ANT SEGMENT INSERTION DRAINAGE W/O RESERVOIR INT 0191T $2,396.00 01967 ANESTH, NEURAXIAL LABOR, PLAN VAG DEL 01967 $2,500.00 01996 PR DAILY MGMT,EPIDUR/SUBARACH CONT DRUG ADM 01996 $285.00 PR PERQ SAC AGMNTJ UNI W/WO BALO/MCHNL DEV 1/> 0200T NDL 0200T $5,106.00 PR PERQ SAC AGMNTJ BI W/WO BALO/MCHNL DEV 2/> 0201T NDLS 0201T $9,446.00 PR INJECT PLATELET RICH PLASMA W/IMG 0232T HARVEST/PREPARATOIN 0232T $1,509.00 0234T PR TRANSLUMINAL PERIPHERAL ATHERECTOMY, RENAL -

Dysphagia: a Symptom Not a Disease
th Case al Re e p Lohe and Kadu, Oral health case Rep 2016, 2:2 H o l r a t r s O Oral Health Case Reports DOI: 10.4172/2471-8726.1000117 ISSN: 2471-8726 ResearchReview Article Article OpenOpen Access Access Dysphagia: A Symptom Not a Disease Vidya K Lohe1* and Ravindra P Kadu2 1Sharad Pawar Dental College and Hospital, DMIMS (DU), Maharashtra, India 2Jawaharlal Nehru Medical College and Hospital DMIMS (DU), Maharashtra, India Abstract Dysphagia is difficulty in swallowing food semi-solid or solid, liquid, or both. There are many disorder conditions predisposing to dysphagia such as mechanical strokes or esophageal diseases even if neurological diseases represent the principal one. Cerebrovascular pathology is today the leading cause of death in developing countries, and it occurs most frequently in individuals who are at least 60 years old. Patients with dysphagia may walk into dental clinic and because dysphagia is a symptom not a disease, it is a practicing dentist’s duty to recognize the underlying cause and then take treatment decisions. Among the most frequent complications of dysphagia are increased mortality and aspiration pneumonia, dehydration, malnutrition, and long-term hospitalization. This review article discusses the pathophysiology, classification, evaluation, investigations and treatment modalities of dysphagia. Keywords: Oropharyngeal dysphagia; Oesophageal dysphagia; Classification of Dysphagia Barium swallow • Oropharyngeal dysphagia is usually described as the inability to Introduction initiate the act of swallowing. It is a “transfer” problem of impaired ability to move food from the mouth into the upper esophagus. It is Phagia meaning swallowing and dysphagia means difficulty in caused by weakness of tongue muscles. -
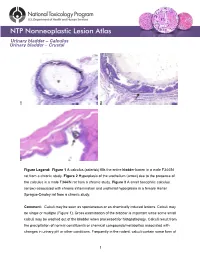
Urinary Bladder – Calculus Urinary Bladder – Crystal
Urinary bladder – Calculus Urinary bladder – Crystal Figure Legend: Figure 1 A calculus (asterisk) fills the entire bladder lumen in a male F344/N rat from a chronic study. Figure 2 Hyperplasia of the urothelium (arrow) due to the presence of the calculus in a male F344/N rat from a chronic study. Figure 3 A small basophilic calculus (arrow) associated with chronic inflammation and urothelial hyperplasia in a female Harlan Sprague-Dawley rat from a chronic study. Comment: Calculi may be seen as spontaneous or as chemically induced lesions. Calculi may be single or multiple (Figure 1). Gross examination of the bladder is important since some small calculi may be washed out of the bladder when processed for histopathology. Calculi result from the precipitation of normal constituents or chemical compounds/metabolites associated with changes in urinary pH or other conditions. Frequently in the rodent, calculi contain some form of 1 Urinary bladder – Calculus Urinary bladder – Crystal calcium or mineral complex. Calculi often result in necrosis, ulceration, inflammation, and hyperplasia of the urothelium (Figure 2 and Figure 3). They are often the cause of bladder obstruction. In addition, bladder neoplasia may result from the presence of calculi. The presence of crystals and the subsequent appearance of calculi are often associated. Strain differences in the presence of crystals have been reported. Crystals, like calculi, tend to be washed out during histologic processing. Recommendation: Calculi and crystals should be diagnosed but should not be graded. Calculi are usually associated with secondary lesions, such as hemorrhage and inflammation. The pathologist should use his or her judgment in deciding whether or not these secondary lesions are prominent enough to warrant a separate diagnosis.