Arc-Staghorn-Calculi.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Urinary Stone Disease – Assessment and Management
Urology Urinary stone disease Finlay Macneil Simon Bariol Assessment and management Data from the Australian Institute of Health and Welfare Background showed an annual incidence of 131 cases of upper urinary Urinary stones affect one in 10 Australians. The majority tract stone disease per 100 000 population in 2006–2007.1 of stones pass spontaneously, but some conditions, particularly ongoing pain, renal impairment and infection, An upper urinary tract stone is the usual cause of what is mandate intervention. commonly called ‘renal colic’, although it is more technically correct to call the condition ‘ureteric colic’. Objective This article explores the role of the general practitioner in Importantly, the site of the pain is notoriously inaccurate in predicting the assessment and management of urinary stones. the site of the stone, except in the setting of new onset lower urinary Discussion tract symptoms, which may indicate distal migration of a stone. The The assessment of acute stone disease should determine majority of stones only become clinically apparent when they migrate the location, number and size of the stone(s), which to the ureter, although many are also found on imaging performed for influence its likelihood of spontaneous passage. Conservative other reasons.2,3 The best treatment of a ureteric stone is frequently management, with the addition of alpha blockers to facilitate conservative (nonoperative), because all interventions (even the more passage of lower ureteric stones, should be attempted in modern ones) carry risks. However, intervention may be indicated in cases of uncomplicated renal colic. Septic patients require urgent drainage and antibiotics. Other indications for referral certain situations. -

The History of Lithotomy and Lithotrity
THE HISTORY OF LITHOTOMY AND LITHOTRITY Arnott Demonstration delivered at the Royal College of Surgeons of England on 24th January 1967 by Sir Eric Riches, M.C., M.S., F.R.C.S. Honorary Curator of Historical Surgical Instruments JAMES MONCRIEFF ARNOTT, who endowed these demonstrations in 1850 during the year of his first Presidency of the College, was elected to the staff of the Middlesex Hospital in 1831, and was one of the founders of its Medical School in 1835. He was chief amongst those who insisted on an eight-day holiday for medical students from Christmas Day to New Year's Day inclusive. An early advocate of the need for specialization in surgery, he undertook in 1843 the duty of running an ophthalmological out-patient clinic in addition to his general surgery. He was a strict disciplinarian but had a dry sense of humour. It was recorded that on a teaching round, after showing a newly invented instrument most completely fitted for the desired purpose, he ended by saying, ' In fact, gentlemen, it is one of those ingenious conceptions which is of no use.' There are many ingenious conceptions in the collection of Historical Surgical Instruments in this College; some are now ' of no use', but many are the precursors of modern instruments and serve as historical landmarks in the development of surgical technique. In no branch is this more evident than in the surgery of stone in the bladder and it seems appropriate to base this account of the history of the operations devised for it on the instruments used. -

Acute Onset Flank Pain-Suspicion of Stone Disease (Urolithiasis)
Date of origin: 1995 Last review date: 2015 American College of Radiology ® ACR Appropriateness Criteria Clinical Condition: Acute Onset Flank Pain—Suspicion of Stone Disease (Urolithiasis) Variant 1: Suspicion of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 8 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). US color Doppler kidneys and bladder 6 O retroperitoneal Radiography intravenous urography 4 ☢☢☢ MRI abdomen and pelvis without IV 4 MR urography. O contrast MRI abdomen and pelvis without and with 4 MR urography. O IV contrast This procedure can be performed with US X-ray abdomen and pelvis (KUB) 3 as an alternative to NCCT. ☢☢ CT abdomen and pelvis with IV contrast 2 ☢☢☢ *Relative Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate Radiation Level Variant 2: Recurrent symptoms of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 7 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated in an emergent setting for acute management to evaluate for hydronephrosis. For planning and US color Doppler kidneys and bladder 7 intervention, US is generally not adequate O retroperitoneal and CT is complementary as CT more accurately characterizes stone size and location. This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). -

Management of Ureteral Stones
Management of Ureteral Stones Ureteral stone disease is among the most painful and prevalent of urologic disorders. As many as 5 percent of Americans will be affected by urinary stones at some point in their lives. Fortunately, most stones pass out of the body without any intervention. If you are not so lucky, the following information should help you and your doctor address the causes, symptoms and possible complications created by your ureteral stone disease. How does the urinary tract work under normal conditions? The urinary tract is similar to a plumbing system, with special pipes that allow water and salts to flow through them. The urinary tract includes two kidneys, two ureters and the urethra. The kidneys act as a filter system for the blood, cleansing it of poisonous materials and retaining valuable glucose, salts and minerals. Urine, the waste product of the filtration, is produced in the kidney and trickles down hours a day through two 10- to 12-inch long tubes called ureters, which connect the kidneys to the bladder. The ureters are about one-fourth inch in diameter and their muscular walls contract to make waves of movement to force the urine into the bladder. The bladder is expandable and stores the urine until it can be conveniently disposed of. It also closes passageways into the ureters so that urine cannot flow back into the kidneys. The tube through which the urine flows out of the body is called the urethra. What is a ureteral stone? A ureteral stone is a kidney stone that has moved down into the ureter. -

Study of Calculus Pancreatitis
STUDY OF CALCULUS PANCREATITIS Dissertation Submitted for MS Degree (Branch I) General Surgery April 2011 The Tamilnadu Dr.M.G.R.Medical University Chennai – 600 032. MADURAI MEDICAL COLLEGE, MADURAI. CERTIFICATE This is to certify that this dissertation titled “STUDY OF CALCULUS PANCREATITIS” submitted by DR.P.K.PRABU to the faculty of General Surgery, The Tamilnadu Dr. M.G.R. Medical University, Chennai in partial fulfillment of the requirement for the award of MS degree Branch I General Surgery, is a bonafide research work carried out by him under our direct supervision and guidance from October 2008 to October 2010. DR. M.GOPINATH, M.S., Pro. A.SANKARAMAHALINGAM M.S, PROFESSOR AND HEAD, PROFESSOR, DEPARTMENT OF GENERAL SURGERY, DEPARTMENT OF GENERAL SURGERY, MADURAI MEDICAL COLLEGE, MADURAI MEDICAL COLLEGE, MADURAI. MADURAI. DECLARATION I, DR.P.K.PRABU solemnly declare that the dissertation titled “STUDY OF CALCULUS PANCREATITIS” has been prepared by me. This is submitted to The Tamilnadu Dr. M.G.R. Medical University, Chennai, in partial fulfillment of the regulations for the award of MS degree (Branch I) General Surgery. Place: Madurai DR. P.K.PRABU Date: ACKNOWLEDGEMENT At the very outset I would like to thank Dr.A.EDWIN JOE M.D.,(FM) the Dean Madurai Medical College and Dr.S.M.SIVAKUMAR M.S., (General Surgery) Medical Superintendent, Government Rajaji Hospital, Madurai for permitting me to carryout this study in this Hospital. I wish to express my sincere thanks to my Head of the Department of Surgery Prof.Dr.M.GOPINATH M.S., and Prof.Dr.MUTHUKRISHNAN M.Ch., Head of the Department of Surgical Gastroenterology for his unstinted encouragement and valuable guidance during this study. -

Flexible Ureteroscopic Laser Lithotripsy for Upper Urinary Tract Stone Disease in Patients with Spinal Cord Injury
Urolithiasis DOI 10.1007/s00240-015-0786-0 ORIGINAL PAPER Flexible ureteroscopic laser lithotripsy for upper urinary tract stone disease in patients with spinal cord injury Abdulkadir Tepeler1 · Brian C. Sninsky1 · Stephen Y. Nakada1 Received: 12 January 2015 / Accepted: 12 May 2015 © Springer-Verlag Berlin Heidelberg 2015 Abstract The objective of this study is to present the hyperuricosuria (n: 1) were common abnormalities in 24-h outcomes of flexible ureteroscopic laser lithotripsy (URS) urine analysis. Ureteroscopic laser lithotripsy can be an for upper urinary tract stone disease in spinal cord injury effective treatment modality for SCI patients with upper (SCI) patients performed by a single surgeon. A retrospec- urinary tract calculi. tive analysis was performed for SCI patients treated with flexible URS for proximal ureter and kidney stone disease Keywords Spinal cord injury · Urolithiasis · by a single surgeon between 2003 and 2013. Patient char- Ureteroscopy · Laser lithotripsy acteristics, operative outcomes, metabolic evaluation, and stone analyses were assessed in detail. A total of 27 URS procedures were performed for urolithiasis in 21 renal Introduction units of 19 patients. The mean age was 52.1 15.6 years ± (16–72) and mean BMI was 29.2 7.3 kg/m2 (20–45.7). Spinal cord injury (SCI) causes neurologic problems ± Etiology of SCI was trauma (n: 10), multiple sclerosis (n: including deterioration of sensorial, motor and autonomic 6), cerebrovascular accident (n: 1), or undetermined (n: 2). functions, leading to restricted physical activity, bladder The mean stone size was 15.9 8.6 (6–40) mm. In the and bowel dysfunction, and metabolic alterations. -

Calcium Kidney Stones Are Associated with Increased Risk Of
Journal of Clinical Medicine Article Calcium Kidney Stones are Associated with Increased Risk of Carotid Atherosclerosis: The Link between Urinary Stone Risks, Carotid Intima-Media Thickness, and Oxidative Stress Markers Ho Shiang Huang 1,2, Pao Chi Liao 3 and Chan Jung Liu 1,* 1 Department of Urology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan 70403, Taiwan; [email protected] 2 Department of Urology, College of Medicine, National Cheng Kung University, Tainan 70403, Taiwan 3 Department of Environmental and Occupational Health, Medical College, National Cheng Kung University, Tainan 70403, Taiwan; [email protected] * Correspondence: [email protected]; Tel.: +886-6-235-3535 (ext. 5251); Fax: +886-6-276-6179 Received: 7 February 2020; Accepted: 6 March 2020; Published: 8 March 2020 Abstract: Previous studies have suggested that kidney stone formers are associated with a higher risk of cardiovascular events. To our knowledge, there have been no previous examinations of the relationship between carotid intima-media thickness (IMT) and urinary stone risk factors. This study was aimed toward an investigation of the association between dyslipidemia, IMT, and 24-hour urinalysis in patients with calcium oxalate (CaOx) or calcium phosphate (CaP) stones. We prospectively enrolled 114 patients with kidney stones and 33 controls between January 2016 and August 2016. All patients were divided into four groups, according to the stone compositions—CaOx 50% group, CaP group, struvite group, and uric acid stones group. Carotid IMT and the carotid ≥ score (CS) were evaluated using extracranial carotid artery doppler ultrasonography. The results of a multivariate analysis indicated that a higher serum total cholesterol (TC) and low-density lipoprotein (LDL) were all associated with lower urinary citrate and higher CS in both the CaOx 50% and CaP ≥ groups. -

Postoperative Intrahepatic Calculus: the Role of Extracorporeal Shockwave Lithotripsy
Published online: 2021-04-10 Case Report Postoperative Intrahepatic Calculus: The Role of Extracorporeal Shockwave Lithotripsy Abstract Asad Irfanullah, Bile duct stones are a known complication after a Roux-en-Y hepaticojejunostomy. Different minimally Kamran Masood, invasive stone extraction techniques, including endoscopic retrograde cholangiopancreatography with Yousuf Memon, basket removal or the use of a choledocoscope through a mature T-tube tract, can be used. However, in some cases, they are unsuccessful due to complicated postsurgical anatomy or technical difficulty. Zakariya Irfanullah In this report, we present a case where extracorporeal shockwave lithotripsy was used in conjunction Department of Radiology, Indus with standard interventional techniques to treat bile duct stones. Hospital, Karachi, Pakistan Keywords: Biliary tract calculus, extracorporeal shockwave lithotripsy, post‑Roux‑en‑y hepaticojejunostomy Introduction medical history was significant for an open cholecystectomy complicated by Bile duct stones and anastomotic strictures iatrogenic injury to the common bile duct are known complications of Roux-en-y and subsequent creation of a Roux-en-y hepaticojejunostomy. Due to the postsurgical hepaticojejunostomy (REHJ). A magnetic anatomy, conventional endoscopic resonance cholangiopancreatography was retrograde cholangiopancreatography performed which demonstrated a significant (ERCP) techniques are often not possible. intrahepatic biliary dilatation with the In this specific case, we treated a large bile formation -

UNJ Dec 2005-427.Ps 11/29/05 3:43 PM Page 427
UNJ Dec 2005-427.ps 11/29/05 3:43 PM Page 427 C Urolithiasis/Nephrolithiasis: O N What’s It All About? T I Joan Colella Bernadette Galli N Eileen Kochis Ravi Munver U I N G he term nephrolithiasis Urolithiasis (urinary tract calculi or stones) and nephrolithiasis (kid- (kidney calculi or stones) ney calculi or stones) are well-documented common occurrences in refers to the entire clini- the general population of the United States. The etiology of this disor- E cal picture of the forma- der is mutifactorial and is strongly related to dietary lifestyle habits or D Ttion and passage of crystal agglom- practices. Proper management of calculi that occur along the urinary U erates called calculi or stones in tract includes investigation into causative factors in an effort to pre- the urinary tract (Wolf, 2004). vent recurrences. Urinary calculi or stones are the most common C Urolithiasis (urinary calculi or cause of acute ureteral obstruction. Approximately 1 in 1,000 adults in A stones) refers to calcifications that the United States are hospitalized annually for treatment of urinary T form in the urinary system, pri- tract stones, resulting in medical costs of approximately $2 billion per marily in the kidney (nephrolithi- I year (Ramello, Vitale, & Marangella, 2000; Tanagho & McAninch, 2004). asis) or ureter (ureterolithiasis), O and may also form in or migrate N into the lower urinary system (bladder or urethra) (Bernier, 2005). Urinary tract stone disease the rest of the world. Researchers Kidney stones are most has been documented historically attribute the incidence of prevalent between the ages of 20 as far back as the Egyptian mum- nephrolithiasis in the United to 40, and a substantial number mies (Wolf, 2004). -

Overtreatment and Underutilization of Watchful Waiting in Men with Limited
ARTICLE IN PRESS Oncology Overtreatment and Underutilization of Watchful Waiting in Men With Limited Life Expectancy: An Analysis of the Michigan Urological Surgery Improvement Collaborative Registry Udit SinghalC, Jeffrey J. TosoianC, Ji Qi, David C. Miller, Susan M. Linsell, Michael Cher, Brian Lane, Michael Cotant, James E. Montie, Wassim Bazzi, Mohammad Jafri, Bradley Rosenberg, and Arvin K. George, Michigan Urological Surgery Improvement Collaborative OBJECTIVE To determine rates of watchful waiting (WW) vs treatment in prostate cancer (PCa) and limited life expectancy (LE) and assess determinants of management. MATERIALS AND Patients diagnosed with PCa between 2012 and 2018 with <10 years LE were identified from the METHODS Michigan Urologic Surgery Improvement Collaborative registry. Multinomial logistic regression models were used to identify factors associated with management choice among NCCN low-risk PCa patients. Data from high-volume practices were analyzed to understand practice variation. RESULTS Total 2393 patients were included. Overall, WW was performed in 8.1% compared to 23.3%, 25%, 11.2%, and 3.6% who underwent AS, radiation (XRT), prostatectomy (RP), and brachytherapy (BT), respectively. In men with NCCN low-risk disease (n = 358), WW was performed in 15.1%, compared to AS (69.3%), XRT (4.2%), RP (6.7%), and BT (2.5%). There was wide variation in management among practices in low-risk men; WW (6%-35%), AS (44%-81%), and definitive treatment (0%-30%). Older age was associated with less likelihood of undergoing AS vs WW (odds ratio [OR] 0.88, P < .001) or treatment vs WW (OR 0.83, P < .0001). Presence of ≥cT2 disease (OR 8.55, P = .014) and greater number of positive biopsy cores (OR 1.41, P = .014) was associated with greater likelihood of treatment vs WW and Charlson comorbidity score of 1 vs 0 (OR 0.23, P = .043) was associated with less likelihood of treatment vs WW. -
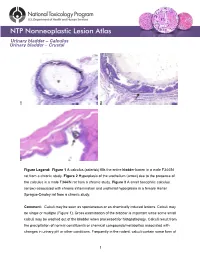
Urinary Bladder – Calculus Urinary Bladder – Crystal
Urinary bladder – Calculus Urinary bladder – Crystal Figure Legend: Figure 1 A calculus (asterisk) fills the entire bladder lumen in a male F344/N rat from a chronic study. Figure 2 Hyperplasia of the urothelium (arrow) due to the presence of the calculus in a male F344/N rat from a chronic study. Figure 3 A small basophilic calculus (arrow) associated with chronic inflammation and urothelial hyperplasia in a female Harlan Sprague-Dawley rat from a chronic study. Comment: Calculi may be seen as spontaneous or as chemically induced lesions. Calculi may be single or multiple (Figure 1). Gross examination of the bladder is important since some small calculi may be washed out of the bladder when processed for histopathology. Calculi result from the precipitation of normal constituents or chemical compounds/metabolites associated with changes in urinary pH or other conditions. Frequently in the rodent, calculi contain some form of 1 Urinary bladder – Calculus Urinary bladder – Crystal calcium or mineral complex. Calculi often result in necrosis, ulceration, inflammation, and hyperplasia of the urothelium (Figure 2 and Figure 3). They are often the cause of bladder obstruction. In addition, bladder neoplasia may result from the presence of calculi. The presence of crystals and the subsequent appearance of calculi are often associated. Strain differences in the presence of crystals have been reported. Crystals, like calculi, tend to be washed out during histologic processing. Recommendation: Calculi and crystals should be diagnosed but should not be graded. Calculi are usually associated with secondary lesions, such as hemorrhage and inflammation. The pathologist should use his or her judgment in deciding whether or not these secondary lesions are prominent enough to warrant a separate diagnosis. -

Obstruction of the Urinary Tract 2567
Chapter 540 ◆ Obstruction of the Urinary Tract 2567 Table 540-1 Types and Causes of Urinary Tract Obstruction LOCATION CAUSE Infundibula Congenital Calculi Inflammatory (tuberculosis) Traumatic Postsurgical Neoplastic Renal pelvis Congenital (infundibulopelvic stenosis) Inflammatory (tuberculosis) Calculi Neoplasia (Wilms tumor, neuroblastoma) Ureteropelvic junction Congenital stenosis Chapter 540 Calculi Neoplasia Inflammatory Obstruction of the Postsurgical Traumatic Ureter Congenital obstructive megaureter Urinary Tract Midureteral structure Jack S. Elder Ureteral ectopia Ureterocele Retrocaval ureter Ureteral fibroepithelial polyps Most childhood obstructive lesions are congenital, although urinary Ureteral valves tract obstruction can be caused by trauma, neoplasia, calculi, inflam- Calculi matory processes, or surgical procedures. Obstructive lesions occur at Postsurgical any level from the urethral meatus to the calyceal infundibula (Table Extrinsic compression 540-1). The pathophysiologic effects of obstruction depend on its level, Neoplasia (neuroblastoma, lymphoma, and other retroperitoneal or pelvic the extent of involvement, the child’s age at onset, and whether it is tumors) acute or chronic. Inflammatory (Crohn disease, chronic granulomatous disease) ETIOLOGY Hematoma, urinoma Ureteral obstruction occurring early in fetal life results in renal dys- Lymphocele plasia, ranging from multicystic kidney, which is associated with ure- Retroperitoneal fibrosis teral or pelvic atresia (see Fig. 537-2 in Chapter 537), to various