A Comprehensive Reference Genome Representation And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Proquest Dissertations
INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. Photographs included in the original manuscript have been reproduced xerographically in this copy. Higher quality 6” x 9” black and white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. UMI Bell & Howell Information and Learning 300 North Zeeb Road, Ann Arbor, Ml 48106-1346 USA 800-521-0600 NOTE TO USERS Page(s) missing in number only; text follows. Microfilmed as received. 222-229 This reproduction is the best copy available. UMI MOLECULAR GENETIC AND BIOCHEMICAL STUDIES OF THE HUMAN AND MOUSE MHC COMPLEMENT GENE CLUSTERS DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Zhenyu Yang, M.S. -

Aneuploidy: Using Genetic Instability to Preserve a Haploid Genome?
Health Science Campus FINAL APPROVAL OF DISSERTATION Doctor of Philosophy in Biomedical Science (Cancer Biology) Aneuploidy: Using genetic instability to preserve a haploid genome? Submitted by: Ramona Ramdath In partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biomedical Science Examination Committee Signature/Date Major Advisor: David Allison, M.D., Ph.D. Academic James Trempe, Ph.D. Advisory Committee: David Giovanucci, Ph.D. Randall Ruch, Ph.D. Ronald Mellgren, Ph.D. Senior Associate Dean College of Graduate Studies Michael S. Bisesi, Ph.D. Date of Defense: April 10, 2009 Aneuploidy: Using genetic instability to preserve a haploid genome? Ramona Ramdath University of Toledo, Health Science Campus 2009 Dedication I dedicate this dissertation to my grandfather who died of lung cancer two years ago, but who always instilled in us the value and importance of education. And to my mom and sister, both of whom have been pillars of support and stimulating conversations. To my sister, Rehanna, especially- I hope this inspires you to achieve all that you want to in life, academically and otherwise. ii Acknowledgements As we go through these academic journeys, there are so many along the way that make an impact not only on our work, but on our lives as well, and I would like to say a heartfelt thank you to all of those people: My Committee members- Dr. James Trempe, Dr. David Giovanucchi, Dr. Ronald Mellgren and Dr. Randall Ruch for their guidance, suggestions, support and confidence in me. My major advisor- Dr. David Allison, for his constructive criticism and positive reinforcement. -

Datasheet Blank Template
SAN TA C RUZ BI OTEC HNOL OG Y, INC . DOM3Z (B-12): sc-393141 BACKGROUND APPLICATIONS DOM3Z (dom-3 homolog Z), also known as NG6 or DOM3L, is a 396 amino DOM3Z (B-12) is recommended for detection of DOM3Z of mouse, rat and acid ubiquitously expressed protein belonging to the DOM3Z family. The gene human origin by Western Blotting (starting dilution 1:100, dilution range encoding DOM3Z maps to human chromosome 6 in the major histocompati - 1:100-1:1000), immunoprecipitation [1-2 µg per 100-500 µg of total protein bility complex (MHC) class III region. Chromosome 6 contains 170 million base (1 ml of cell lysate)], immunofluorescence (starting dilution 1:50, dilution pairs and comprises nearly 6% of the human genome. Deletion of a portion range 1:50-1:500), immunohistochemistry (including paraffin-embedded of the q arm of chromosome 6 is associated with early onset intestinal can - sections) (starting dilution 1:50, dilution range 1:50-1:500) and solid phase cer, suggesting the presence of a cancer susceptibility locus. Additionally, ELISA (starting dilution 1:30, dilution range 1:30-1:3000). Porphyria cutanea tarda, Parkinson’s disease and Stickler syndrome are all Suitable for use as control antibody for DOM3Z siRNA (h): sc-95321, DOM3Z associated with genes that map to chromosome 6. siRNA (m): sc-143143, DOM3Z shRNA Plasmid (h): sc-95321-SH, DOM3Z shRNA Plasmid (m): sc-143143-SH, DOM3Z shRNA (h) Lentiviral Particles: REFERENCES sc-95321-V and DOM3Z shRNA (m) Lentiviral Particles: sc-143143-V. 1. Brunner, H.G., et al . -

DNA Methylation Alterations in Blood Cells of Toddlers with Down Syndrome
G C A T T A C G G C A T genes Article DNA Methylation Alterations in Blood Cells of Toddlers with Down Syndrome Oxana Yu. Naumova 1,2,* , Rebecca Lipschutz 2, Sergey Yu. Rychkov 1, Olga V. Zhukova 1 and Elena L. Grigorenko 2,3,4,* 1 Vavilov Institute of General Genetics RAS, 119991 Moscow, Russia; [email protected] (S.Y.R.); [email protected] (O.V.Z.) 2 Department of Psychology, University of Houston, Houston, TX 77204, USA; [email protected] 3 Department of Psychology, Saint-Petersburg State University, 199034 Saint Petersburg, Russia 4 Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, TX 77030, USA * Correspondence: [email protected] or [email protected] (O.Y.N.); [email protected] (E.L.G.) Abstract: Recent research has provided evidence on genome-wide alterations in DNA methylation patterns due to trisomy 21, which have been detected in various tissues of individuals with Down syndrome (DS) across different developmental stages. Here, we report new data on the systematic genome-wide DNA methylation perturbations in blood cells of individuals with DS from a previously understudied age group—young children. We show that the study findings are highly consistent with those from the prior literature. In addition, utilizing relevant published data from two other developmental stages, neonatal and adult, we track a quasi-longitudinal trend in the DS-associated DNA methylation patterns as a systematic epigenomic destabilization with age. Citation: Naumova, O.Y.; Lipschutz, R.; Rychkov, S.Y.; Keywords: Down syndrome; infants and toddlers; trisomy 21; DNA methylation; Illumina 450K Zhukova, O.V.; Grigorenko, E.L. -

UNIVERSITY of CALIFORNIA Los Angeles the Subproteome Of
UNIVERSITY OF CALIFORNIA Los Angeles The subproteome of mitoBKCa channels from cardiomyocytes reveals novel insights into its mitochondrial import mechanism and function A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Molecular and Medical Pharmacology by Jin Zhang 2016 © Copyright by Jin Zhang 2016 ABSTRACT OF DISSERTATION The subproteome of mitoBKCa channels from cardiomyocytes reveals novel insights into its mitochondrial import mechanism and function by Jin Zhang Doctor of Philosophy in Molecular and Medical Pharmacology University of California, Los Angeles, 2016 Professor Ligia G Toro De Stefani, CO-Chair Professor Riccardo Olcese, CO-Chair BKCa channels are widely expressed ion channels and characterized by their large conductance to potassium and sensitivity to calcium and voltage. It is typically observed at the plasma membrane in the majority of cell types, with adult cardiomyocytes being an exception. Previously, both electrophysiological and immunological studies have detected BKCa channels in + cardiomyocytes mitoplasts, confirming the presence of this K channel (mitoBKCa); however, its physiological functions have not yet fully understood. This work is the first one to examine the interactome of mitoBKCa channels in adult heart (isolated cardiomyocytes and whole ventricle). We used a directed proteomic approach aided by co-immunoprecipitation with BKCa antibodies and pull-down with recombinant DEC sequences. ii As a result, we identified an extensive network of the mitoBKCa, channel and overall, a total of 1079 different proteins were identified as partners of the BKCa channel in cardiomyocytes and left ventricle, including 151 mitochondrial proteins. Two putative protein partners were selected to validate and further examine the associations with BKCa channels: i) the Tom22 from the mitochondrial import system, and ii) the adenine nucleotide translocator (ANT), which is linked to oxidative phosphorylation and the regulation of mPTP. -

Supporting Information
Supporting Information Poulogiannis et al. 10.1073/pnas.1009941107 SI Materials and Methods Loss of Heterozygosity (LOH) Analysis of PARK2. Seven microsatellite Bioinformatic Analysis of Genome and Transcriptome Data. The markers (D6S1550, D6S253, D6S305, D6S955, D6S980, D6S1599, aCGH package in R was used to identify significant DNA copy and D6S396) were amplified for LOH analysis within the PARK2 number (DCN) changes in our collection of 100 sporadic CRCs locus using primers that were previously described (8). (1) (Gene Expression Omnibus, accession no. GSE12520). The MSP of the PARK2 Promoter. CpG sites within the PARK2 promoter aCGH analysis of cell lines and liver metastases was derived region were detected using the Methprimer software (http://www. from published data (2, 3). Chromosome 6 tiling-path array- urogene.org/methprimer/index.html). Methylation-specificand CGH was used to identify the smallest and most frequently al- control primers were designed using the Primo MSP software tered regions of DNA copy number change on chromosome 6. (http://www.changbioscience.com/primo/primom.html); bisulfite An integrative approach was used to correlate expression pro- modification of genomic DNA was performed as described pre- files with genomic copy number data from a SNP array from the viously (9). All tumor DNA samples from primary CRC tumors same tumors (n = 48) (4) (GSE16125), using Pearson’s corre- (n = 100) and CRC lines (n = 5), as well as those from the leukemia lation coefficient analysis to identify the relationships between cell lines KG-1a (acute myeloid leukemia, AML), U937 (acute DNA copy number changes and gene expression of those genes lymphoblastic leukemia, ALL), and Raji (Burkitt lymphoma, BL) SssI located within the small frequently altered regions of DCN were screened as part of this analysis. -

DOM3Z (DXO) (NM 005510) Human Recombinant Protein Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for TP305657 DOM3Z (DXO) (NM_005510) Human Recombinant Protein Product data: Product Type: Recombinant Proteins Description: Recombinant protein of human dom-3 homolog Z (C. elegans) (DOM3Z) Species: Human Expression Host: HEK293T Tag: C-Myc/DDK Predicted MW: 44.7 kDa Concentration: >50 ug/mL as determined by microplate BCA method Purity: > 80% as determined by SDS-PAGE and Coomassie blue staining Buffer: 25 mM Tris.HCl, pH 7.3, 100 mM glycine, 10% glycerol Preparation: Recombinant protein was captured through anti-DDK affinity column followed by conventional chromatography steps. Storage: Store at -80°C. Stability: Stable for 12 months from the date of receipt of the product under proper storage and handling conditions. Avoid repeated freeze-thaw cycles. RefSeq: NP_005501 Locus ID: 1797 UniProt ID: O77932, A0A024RCW8 RefSeq Size: 1590 Cytogenetics: 6p21.33 RefSeq ORF: 1188 Synonyms: DOM3L; DOM3Z; NG6; RAI1 Summary: This gene localizes to the major histocompatibility complex (MHC) class III region on chromosome 6. The function of its protein product is unknown, but its ubiquitous expression and conservation in both simple and complex eukaryotes suggests that this may be a housekeeping gene. [provided by RefSeq, Jul 2008] This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene Technologies, Inc., 9620 Medical Center Drive, Ste 200, Rockville, MD 20850, US 1 / 2 DOM3Z (DXO) (NM_005510) Human Recombinant Protein – TP305657 Product images: Coomassie blue staining of purified DXO protein (Cat# TP305657). -

Predict AID Targeting in Non-Ig Genes Multiple Transcription Factor
Downloaded from http://www.jimmunol.org/ by guest on September 26, 2021 is online at: average * The Journal of Immunology published online 20 March 2013 from submission to initial decision 4 weeks from acceptance to publication Multiple Transcription Factor Binding Sites Predict AID Targeting in Non-Ig Genes Jamie L. Duke, Man Liu, Gur Yaari, Ashraf M. Khalil, Mary M. Tomayko, Mark J. Shlomchik, David G. Schatz and Steven H. Kleinstein J Immunol http://www.jimmunol.org/content/early/2013/03/20/jimmun ol.1202547 Submit online. Every submission reviewed by practicing scientists ? is published twice each month by http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://www.jimmunol.org/content/suppl/2013/03/20/jimmunol.120254 7.DC1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2013 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 26, 2021. Published March 20, 2013, doi:10.4049/jimmunol.1202547 The Journal of Immunology Multiple Transcription Factor Binding Sites Predict AID Targeting in Non-Ig Genes Jamie L. Duke,* Man Liu,†,1 Gur Yaari,‡ Ashraf M. Khalil,x Mary M. Tomayko,{ Mark J. Shlomchik,†,x David G. -
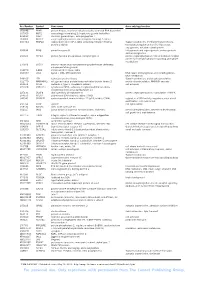
For Personal Use. Only Reproduce with Permission from the Lancet Publishing Group
Acc Number Symbol Gene name Gene ontology function U50648 PRKR protein kinase, interferon-inducible double stranded RNA dependent U37055 MST1 macrophage stimulating 1 (hepatocyte growth factor-like) K03183 CGB7 chorionic gonadotropin, beta polypeptide 7 J03069 MYCL2 v-myc myelocytomatosis viral oncogene homolog 2 (avian) M36711 TFAP2A transcription factor AP-2 alpha (activating enhancer binding Signal transduction, developmental processes, protein 2 alpha) transcription regulation from Pol II promoter, oncogenesis, ectoderm development X69699 PAX8 paired box gene 8 histogenesis and organogenesis, embryogenesis and morphogenesis L38929 PTPRD protein tyrosine phosphatase, receptor type, D protein dephosphorylation, transmembrane receptor protein tyrosine phosphatase signalling, phosphate metabolism L76568 ERCC4 excision repair cross-complementing rodent repair deficiency, complementation group 4 U11870 IL8RA interleukin 8 receptor, alpha M36067 LIG1 ligase I, DNA, ATP-dependent DNA repair, embryogenesis and morphogenesis, DNA metabolism D16105 LTK leukocyte tyrosine kinase Signal transduction, protein phosphorylation U12779 MAPKAPK2 mitogen-activated protein kinase-activated protein kinase 2 protein phosphorylation, MAPKKK cascade L34059 CDH4 cadherin 4, type 1, R-cadherin (retinal) cell adhesion U22028 CYP2A13 cytochrome P450, subfamily IIA (phenobarbital-inducible), complementation group 4polypeptide 13 U27193 DUSP8 dual specificity phosphatase 8 protein dephosphorylation, inactiviation of MAPK L24559 POLA2 polymerase (DNA-directed), alpha -

Evaluation of Proteomic and Transcriptomic Biomarker Discovery
EVALUATION OF PROTEOMIC AND TRANSCRIPTOMIC BIOMARKER DISCOVERY TECHNOLOGIES IN OVARIAN CANCER. CLARE RITA ELIZABETH COVENEY A thesis submitted in partial fulfilment of the requirements of the Nottingham Trent University for the degree of Doctor of Philosophy October 2016 Copyright Statement “This work is the intellectual property of the author. You may copy up to 5% of this work for private study, or personal, non-commercial research. Any re-use of the information contained within this document should be fully referenced, quoting the author, title, university, degree level and pagination. Queries or requests for any other use, or if a more substantial copy is required, should be directed in the owner(s) of the Intellectual Property Rights.” Acknowledgments This work was funded by The John Lucille van Geest Foundation and undertaken at the John van Geest Cancer Research Centre, at Nottingham Trent University. I would like to extend my foremost gratitude to my supervisory team Professor Graham Ball, Dr David Boocock, Professor Robert Rees for their guidance, knowledge and advice throughout the course of this project. I would also like to show my appreciation of the hard work of Mr Ian Scott, Professor Bob Shaw and Dr Matharoo-Ball, Dr Suman Malhi and later Mr Viren Asher who alongside colleagues at The Nottingham University Medical School and Derby City General Hospital initiated the ovarian serum collection project that lead to this work. I also would like to acknowledge the work of Dr Suha Deen at Queen’s Medical Centre and Professor Andrew Green and Christopher Nolan of the Cancer & Stem Cells Division of the School of Medicine, University of Nottingham for support with the immunohistochemistry. -

Biomarkers for Lupus
(19) TZZ ¥ _T (11) EP 2 375 252 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 12.10.2011 Bulletin 2011/41 G01N 33/564 (2006.01) (21) Application number: 11158716.8 (22) Date of filing: 11.06.2009 (84) Designated Contracting States: • Fallon, Rachel, Alison AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Maidenhead, Berkshire SL6 7RJ (GB) HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL • Joyce, Sarah, Paula PT RO SE SI SK TR Maidenhead, Berkshire SL6 7RJ (GB) • Koopman, Jens-Oliver (30) Priority: 11.06.2008 GB 0810709 Maidenhead, Berkshire SL6 7RJ (GB) • McAndrew, Michael, Bernard (62) Document number(s) of the earlier application(s) in Maidenhead, Berkshire SL6 7RJ (GB) accordance with Art. 76 EPC: • Workman,, Nicholas, Ian 09761971.2 / 2 300 826 Maidenhead, Berkshire SL6 7RJ (GB) (71) Applicant: SENSE PROTEOMIC LIMITED (74) Representative: Marshall, Cameron John Yarnton Carpmaels & Ransford Oxford One Southampton Row Oxfordshire OX5 1PF (GB) London WC1B 5HA (GB) (72) Inventors: Remarks: • Ebner, Martin Johannes This application was filed on 17-03-2011 as a Maidenhead, Berkshire SL6 7RJ (GB) divisional application to the application mentioned • Wheeler, Colin, Henry under INID code 62. Maiadenhead, Berkshire SL6 7RJ (GB) (54) Biomarkers for lupus (57) The present invention provides biomarkers val- the progression or regression of lupus, or monitoring the uable in diagnosing or detecting a susceptibility to lupus. progression to a flare of the disease or transition from a The present invention also provides panels of biomarkers flare into remission or monitoring the efficacy of a thera- valuable in diagnosing or detecting a susceptibility to lu- peutic agent to lupus. -

Sequence Tags for Camelus Dromedarius
Sequencing, Analysis, and Annotation of Expressed Sequence Tags for Camelus dromedarius Abdulaziz M. Al-Swailem1, Maher M. Shehata1, Faisel M. Abu-Duhier1, Essam J. Al-Yamani1, Khalid A. Al-Busadah2, Mohammed S. Al-Arawi1, Ali Y. Al-Khider1, Abdullah N. Al-Muhaimeed1, Fahad H. Al-Qahtani1, Manee M. Manee1, Badr M. Al-Shomrani1, Saad M. Al-Qhtani1, Amer S. Al-Harthi1, Kadir C. Akdemir3, Mehmet S. Inan1{, Hasan H. Otu1,3* 1 Biotechnology Research Center, Natural Resources and Environment Research Institute, King Abdulaziz City for Science and Technology, Riyadh, Saudi Arabia, 2 Faculty of Veterinary Medicine and Animal Resources, King Faisal University, Al-Hassa, Saudi Arabia, 3 Department of Medicine, BIDMC Genomics Center, Harvard Medical School, Boston, Massachusetts, United States of America Abstract Despite its economical, cultural, and biological importance, there has not been a large scale sequencing project to date for Camelus dromedarius. With the goal of sequencing complete DNA of the organism, we first established and sequenced camel EST libraries, generating 70,272 reads. Following trimming, chimera check, repeat masking, cluster and assembly, we obtained 23,602 putative gene sequences, out of which over 4,500 potentially novel or fast evolving gene sequences do not carry any homology to other available genomes. Functional annotation of sequences with similarities in nucleotide and protein databases has been obtained using Gene Ontology classification. Comparison to available full length cDNA sequences and Open Reading Frame (ORF) analysis of camel sequences that exhibit homology to known genes show more than 80% of the contigs with an ORF.300 bp and ,40% hits extending to the start codons of full length cDNAs suggesting successful characterization of camel genes.