Plant Resource Journal 2020 Final.Pmd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogenetic Analyses of Juniperus Species in Turkey and Their Relations with Other Juniperus Based on Cpdna Supervisor: Prof
MOLECULAR PHYLOGENETIC ANALYSES OF JUNIPERUS L. SPECIES IN TURKEY AND THEIR RELATIONS WITH OTHER JUNIPERS BASED ON cpDNA A THESIS SUBMITTED TO THE GRADUATE SCHOOL OF NATURAL AND APPLIED SCIENCES OF MIDDLE EAST TECHNICAL UNIVERSITY BY AYSUN DEMET GÜVENDİREN IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN BIOLOGY APRIL 2015 Approval of the thesis MOLECULAR PHYLOGENETIC ANALYSES OF JUNIPERUS L. SPECIES IN TURKEY AND THEIR RELATIONS WITH OTHER JUNIPERS BASED ON cpDNA submitted by AYSUN DEMET GÜVENDİREN in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Department of Biological Sciences, Middle East Technical University by, Prof. Dr. Gülbin Dural Ünver Dean, Graduate School of Natural and Applied Sciences Prof. Dr. Orhan Adalı Head of the Department, Biological Sciences Prof. Dr. Zeki Kaya Supervisor, Dept. of Biological Sciences METU Examining Committee Members Prof. Dr. Musa Doğan Dept. Biological Sciences, METU Prof. Dr. Zeki Kaya Dept. Biological Sciences, METU Prof.Dr. Hayri Duman Biology Dept., Gazi University Prof. Dr. İrfan Kandemir Biology Dept., Ankara University Assoc. Prof. Dr. Sertaç Önde Dept. Biological Sciences, METU Date: iii I hereby declare that all information in this document has been obtained and presented in accordance with academic rules and ethical conduct. I also declare that, as required by these rules and conduct, I have fully cited and referenced all material and results that are not original to this work. Name, Last name : Aysun Demet GÜVENDİREN Signature : iv ABSTRACT MOLECULAR PHYLOGENETIC ANALYSES OF JUNIPERUS L. SPECIES IN TURKEY AND THEIR RELATIONS WITH OTHER JUNIPERS BASED ON cpDNA Güvendiren, Aysun Demet Ph.D., Department of Biological Sciences Supervisor: Prof. -

Extract of Juniperus Indica Bertol Synergizes with Cisplatin to Inhibit
molecules Article Extract of Juniperus indica Bertol Synergizes with Cisplatin to Inhibit Oral Cancer Cell Growth via Repression of Cell Cycle Progression and Activation of the Caspase Cascade Xiao-Fan Huang 1,2, Kai-Fu Chang 1,2 , Shan-Chih Lee 3,4, Chia-Yu Li 5, Hung-Hsiu Liao 2, Ming-Chang Hsieh 2,6,* and Nu-Man Tsai 2,6,* 1 Institute of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan; [email protected] (X.-F.H.); [email protected] (K.-F.C.) 2 Department of Medical Laboratory and Biotechnology, Chung Shan Medical University, Taichung 40201, Taiwan; [email protected] 3 Department of Medical Imaging and Radiological Sciences, Chung Shan Medical University, Taichung 40201, Taiwan; [email protected] 4 Department of Medical Imaging, Chung Shan Medical University Hospital, Taichung 40201, Taiwan 5 Department of Life and Death, Nanhua University, Chiayi 62249, Taiwan; [email protected] 6 Clinical Laboratory, Chung Shan Medical University Hospital, Taichung 40201, Taiwan * Correspondence: [email protected] (M.-C.H.); [email protected] (N.-M.T.); Tel.: +886-4-2473-0022 (ext. 12411) (N.-M.T.); Fax: +886-4-2324-8171 (N.-M.T.) Academic Editors: Halina Ekiert and Agnieszka Szopa Received: 21 May 2020; Accepted: 12 June 2020; Published: 13 June 2020 Abstract: Oral cancer—a type of head and neck cancer—is estimated to be the fifth most common cancer in Taiwan. However, efficacious therapies for oral cancer are still lacking due to drug resistance and recurrence. Consequently, the identification of new anticancer agents for clinical treatment is needed. -

Himalayan Aromatic Medicinal Plants: a Review of Their Ethnopharmacology, Volatile Phytochemistry, and Biological Activities
medicines Review Himalayan Aromatic Medicinal Plants: A Review of their Ethnopharmacology, Volatile Phytochemistry, and Biological Activities Rakesh K. Joshi 1, Prabodh Satyal 2 and Wiliam N. Setzer 2,* 1 Department of Education, Government of Uttrakhand, Nainital 263001, India; [email protected] 2 Department of Chemistry, University of Alabama in Huntsville, Huntsville, AL 35899, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-256-824-6519; Fax: +1-256-824-6349 Academic Editor: Lutfun Nahar Received: 24 December 2015; Accepted: 3 February 2016; Published: 19 February 2016 Abstract: Aromatic plants have played key roles in the lives of tribal peoples living in the Himalaya by providing products for both food and medicine. This review presents a summary of aromatic medicinal plants from the Indian Himalaya, Nepal, and Bhutan, focusing on plant species for which volatile compositions have been described. The review summarizes 116 aromatic plant species distributed over 26 families. Keywords: Jammu and Kashmir; Himachal Pradesh; Uttarakhand; Nepal; Sikkim; Bhutan; essential oils 1. Introduction The Himalya Center of Plant Diversity [1] is a narrow band of biodiversity lying on the southern margin of the Himalayas, the world’s highest mountain range with elevations exceeding 8000 m. The plant diversity of this region is defined by the monsoonal rains, up to 10,000 mm rainfall, concentrated in the summer, altitudinal zonation, consisting of tropical lowland rainforests, 100–1200 m asl, up to alpine meadows, 4800–5500 m asl. Hara and co-workers have estimated there to be around 6000 species of higher plants in Nepal, including 303 species endemic to Nepal and 1957 species restricted to the Himalayan range [2–4]. -

Variation in Leaf Biomass and Fruit Output of Juniperus Indica Along an Elevation Gradient in North-Central Nepal A
Variation in leaf biomass and fruit output of Juniperus indica along an elevation gradient in north-central Nepal A. Chapagain1, 3, R. P. Chaudhary2 and S. K. Ghimire3 Biomass and reproductive output are important functional traits that influence aspects of plant performance. Measurements of these attributes by harvesting plant parts are often destructive and impractical. Therefore, non-destructive methods, based on allometric relationships, have been recommended for measuring plant biomass and reproductive output, particularly in the ecosystems where plant harvesting is not very practical or feasible. Here, we assessed the variation in the traits related to vegetative and reproductive performance (including plant height, trunk diameter, canopy area, leaf biomass and number of fruits set) among populations of Juniperus indica distributed along an elevation gradient in Manang district of the north-central Nepal, and finally determined the allometric relationships addressing the leaf biomass and the fruit output. The distribution range of J. indica was divided into lower- (3,350‒3,580m), mid- (3,650‒3,880m) and higher- (3,950‒4,250m) elevation classes where we made 54 sample plots of 10m × 10m size. In each plot, we recorded the number of individuals of J. indica classifying into seedling, juvenile and mature classes, and measured their vegetative traits and fruit output. Trunk diameter, leaf dry-weight and fruits set parameters spatially varied within the same elevation class. The individuals at the lower-elevation were larger in vegetative size with larger- trunk, height and canopy area, and produced higher leaf biomass and greater number of fruits as compared to those produced by the individuals situated at the mid- and higher-elevations. -

National Register of Medicinal Plants
Digitized by Google Digitized by Google IUCI Nepal National Register of Medicinal Plants IUCl-The World Conservation union May 2000 ... .....,...... , ... 111 IUCN ....,, ., fllrlll •• ... c-.ltloll n.w.wc:-....u.i. IIHI l111I11111I1111II1111II111111111111111 9AZG-Y9Q-23PK Published by: IUCN Nepal Copyright: 2000. IUCN Nepal The role of Swiss Agency for Development and Cooperation in supporting the IUCN Nepal is gratefully acknowledged. The material in this publication may be reproduced in whole or in part and in any form for education or non-profit uses, without special permission from the copyright holder, provided acknowledgment of the source is made. IUCN Nepal would appreciate receiving a copy of any publication which uses this publication as a source. No use of this publication may be made for resale or other commercial purposes without prior written permission of IUCN Nepal. Citation: IUCN Nepal. 2000. National Register ofMedicinal Plants. Kathmandu: IUCN Nepal. ix+ 163 pp. ISBN: 92-9144-048-5 Layout and Design: Upendra Shrestha & Kanhaiya L. Shrestha Cover design: Upendra Shrestha Cover Pictures: Pages from the manuscript of Chandra Nighantu drawn towards the end of 19th century (Courtesy: Singh Durbar Vaidhyakhana Development Committee) Left-hand side: Rajbriksha (Cassia fistula) occuring in the Tarai and other tropical regions of Nepal lying below 1,000 m altitude. Right-hand side: jatamansi (Nardostachys grandif/ora) occuring at 3,000m to 4,000m in the alpine and subalpine zone of Nepal Himalaya. Available from: IUCN Nepal P.O. Box 3923 Kathmandu, Nepal The views expressed in this document are those of the authors and do not necessarily reflect the official views of IUCN Nepal. -

Plant Ecophysiology
Plant Ecophysiology Michael Tausz Nancy Grulke Editors Trees in a Changing Environment Ecophysiology, Adaptation, and Future Survival Trees in a Changing Environment [email protected] Plant Ecophysiology Volume 9 Series Editors: Luit J. De Kok University of Groningen, The Netherlands Malcolm J. Hawkesford Rothamsted Research, United Kingdom Aims & Scope: The Springer Series in Plant Ecophysiology comprises a series of volumes that deals with the impact of biotic and abiotic factors on plant functioning and physiological adaptation to the environment. The aim of the Plant Ecophysiology series is to review and integrate the present knowledge on the impact of the environment on plant functioning and adaptation at various levels: from the molecular, biochemical and physiological to a whole plant level. This series is of interest to scientists who like to be informed of new developments and insights in plant ecophysiology, and can be used as advanced textbooks for biology students. The titles published in this series are listed at the end of this volume. [email protected] Trees in a Changing Environment Ecophysiology, Adaptation, and Future Survival Edited by Michael Tausz Forest and Ecosystem Science University of Melbourne Victoria Australia and Nancy Grulke Pacific NW Research Station WWETAC USDA Forest Service Prineville, Oregon, USA [email protected] Editors Michael Tausz Nancy Grulke Forest and Ecosystem Science Pacific NW Research Station WWETAC University of Melbourne USDA Forest Service Victoria Prineville, Oregon, USA Australia ISSN 1572-5561 ISSN 1572-5561 (electronic) ISBN 978-94-017-9099-4 ISBN 978-94-017-9100-7 (eBook) DOI 10.1007/978-94-017-9100-7 Springer Dordrecht Heidelberg New York London Library of Congress Control Number: 2014946587 © Springer Science+Business Media Dordrecht (outside the USA) 2014 Chapter 11 was created within the capacity of an US government employment. -

Journal of Threatened Taxa ISSN 0974-7907 (Online) ISSN 0974-7893 (Print)
Journal of Threatened Taxa ISSN 0974-7907 (Online) ISSN 0974-7893 (Print) 26 July 2019 (Online & Print) PLATINUM Vol. 11 | No. 9 | 14087–14246 OPEN 10.11609/jott.2019.11.9.14087-14246 ACCESS www.threatenedtaxa.org J Building TTevidence for conservation globally ISSN 0974-7907 (Online); ISSN 0974-7893 (Print) Publisher Host Wildlife Information Liaison Development Society Zoo Outreach Organization www.wild.zooreach.org www.zooreach.org No. 12, Thiruvannamalai Nagar, Saravanampatti - Kalapatti Road, Saravanampatti, Coimbatore, Tamil Nadu 641035, India Ph: +91 9385339863 | www.threatenedtaxa.org Email: [email protected] EDITORS Typesetting Founder & Chief Editor Mr. Arul Jagadish, ZOO, Coimbatore, India Dr. Sanjay Molur Mrs. Radhika, ZOO, Coimbatore, India Wildlife Information Liaison Development (WILD) Society & Zoo Outreach Organization (ZOO), Mrs. Geetha, ZOO, Coimbatore India 12 Thiruvannamalai Nagar, Saravanampatti, Coimbatore, Tamil Nadu 641035, India Mr. Ravindran, ZOO, Coimbatore India Deputy Chief Editor Fundraising/Communications Dr. Neelesh Dahanukar Mrs. Payal B. Molur, Coimbatore, India Indian Institute of Science Education and Research (IISER), Pune, Maharashtra, India Editors/Reviewers Managing Editor Subject Editors 2016-2018 Mr. B. Ravichandran, WILD, Coimbatore, India Fungi Associate Editors Dr. B.A. Daniel, ZOO, Coimbatore, Tamil Nadu 641035, India Dr. B. Shivaraju, Bengaluru, Karnataka, India Ms. Priyanka Iyer, ZOO, Coimbatore, Tamil Nadu 641035, India Prof. Richard Kiprono Mibey, Vice Chancellor, Moi University, Eldoret, Kenya Dr. Mandar Paingankar, Department of Zoology, Government Science College Gadchiroli, Dr. R.K. Verma, Tropical Forest Research Institute, Jabalpur, India Chamorshi Road, Gadchiroli, Maharashtra 442605, India Dr. V.B. Hosagoudar, Bilagi, Bagalkot, India Dr. Ulrike Streicher, Wildlife Veterinarian, Eugene, Oregon, USA Dr. Vatsavaya S. -

Some High Value Medicinal Plants of Khumbu Region Nepal
Nepal Journal of Science and Technology 10 (2009) 73-82 Some High Value Medicinal Plants of Khumbu Region Nepal Deepa Shree Rawal, Jaishree Sijapati, Neesha Rana, Prabina Rana, Anjana Giri and Sangita Shrestha Nepal Academy of Science and Technology, Khumaltar, Lalitpur, Nepal Abstract Visualizing the present biodiversity status of Nepal, an integrated approach comprising of biodiversity documentation, bio-prospecting, sustainable utilization and conservation has become an urgent need. Realizing the importance of medicinal plants altogether 45 plants were collected from Khumbu region with the altitudinal variation of 2582m to 4470m. Among the collected plant species 12 have been characterized as highly valuable. The important biochemical constituents and morphology of these plants have been described in this paper. Key words: Himalayan region, Khumbu, medicinal plants, morphological characters. Introduction Medicinal plants are second most valuable bio-resources alternative medicines in the form of herbal drugs and of Nepal after water resources. Many highly demanded Ayurvedic medicines. Many such high value medicinal and globally important medicinal plants such as Swertia plants have been equally used in other forms of spp., Neo-picrorhiza scrophulariiflora, Podophyllum alternative medicines such as Chinese, Tibetan and hexandrum, Taxus wallichiana, Podocarpus sp. etc. are Homoeopathic medicine (Li et al. 1997, Dhama & harbored in various geoclimatic region of Nepal (Polunin Dhama 1996). Therefore, the production, consumption & Stainton 1984, Press et al. 2000). The total number of and international trade in medicinal plants and phyto- medicinal and aromatic plants reported in Nepal varies medicines are growing and expected to grow quite according to various authors (DPR 1970, 1984; Malla & significantly in future. -

Identification Key to the Cypress Family (Cupressaceae)1
Feddes Repertorium 116 (2005) 1–2, 96–146 DOI: 10.1002/fedr.200411062 Weinheim, Mai 2005 Ruhr-Universität Bochum, Lehrstuhl für Spezielle Botanik, Bochum C. SCHULZ; P. KNOPF & TH. STÜTZEL Identification key to the Cypress family (Cupressaceae)1 With 11 Figures Summary Zusammenfassung The identification of Cupressaceae taxa, except for Bestimmungsschlüssel für die Familie der Cup- some local and easily distinguishable taxa, is diffi- ressaceae cult even for specialists. One reason for this is the lack of a complete key including all Cupressaceae Die Bestimmung von Cupressaceae-Taxa ist mit taxa, another reason is that diagnoses and descrip- Ausnahme einiger lokaler und leicht bestimmbarer tions are spread over several hundred publications Taxa schwierig, selbst für Spezialisten. Ein Grund, which are sometimes difficult to access. Based on warum es noch keinen vollständigen Bestimmungs- morphological studies of about 3/4 of the species and schlüssel mit allen Cupressaceae-Taxa gibt ist, dass a careful compilation of the most important descrip- die Sippen-Beschreibungen sich auf mehrere hundert tions of Cupressaceae, a first identification key for Publikationen verteilen, welche teilweise schwierig the entire Cypress family (Cupressaceae) could be zu beschaffen sind. Etwa 3/4 der Cupressaceae-Ar- set up. The key comprises any of the 30 genera, 134 ten wurden morphologisch untersucht und die wich- species, 7 subspecies, 38 varieties, one form and thus tigsten Beschreibungen zusammengefasst, daraus all 180 taxa recognized by FARJON (2001). The key wurde dann der erste vollständige Bestimmungs- uses mainly features of adult leaves, female cones schlüssel für Cupressaceae erstellt. Der Bestim- and other characters which are all relatively easy to mungsschlüssel enthält 30 Gattungen, 134 Arten, be used. -

Of Juniperus Recurva
Journal of Experimental and Integrative Medicine www.jeim.org 10.5455/jeim.260416.rw.015 Mini Review DOI: A review on phyto-pharmacological potentials of Juniperus recurva Abila Rashid1, Raja Amir Hassan Kuchay2 1Faculty of Pharmaceutical ABSTRACT Sciences, Shri Venkateshwara University, Gajraula, UP, India. Juniperus recurva is a small tree or spreading shrub distributed from Afghanistan eastward through the Himalayas to 2School of Biosciences & southwest China. It is predominant in woodlands of the wetter areas of Himalayas (altitude of 1800 m to 3900 m), with Biotechnology, CBS, BGSB University, moist and well-drained soils. It holds great potential as a medicinal plant. It has been traditionally used as anti-rheumatic, J&K, India. antiseptic, antispasmodic, antitoxic, aphrodisiac, astringent, carminative, rubificant and stomachic. However, very few authentic pharmacological studies on medicinal benefits of this plant have been conducted thus far. In a first of its kind, Address for correspondence: we review this plant for its chemical constituents, pharmacological activity and medicinal benefits. Keeping in view its Raja A.H Kuchay, success record as a traditional medicine to cure different ailments, it would be of interest to look for various bioactive School of Biosciences & natural products that might have therapeutic potential. Biotechnology, CBS, BGSB University, J&K, India. [email protected] Received: January 12, 2016 Accepted: April 26, 2016 Published: June 30, 2016 KEY WORDS: Juniperus recurva; Distribution; Pharmacological Activity. INTRODUCTION survey. No limits were imposed in terms of author or type of publication. Juniperus L. (family Cupressaceae), a genus of evergreen aromatic shrubs or trees is distributed in temperate and OCCURRENCE AND MORPHOLOGY cold regions of northern Hemisphere [1]. -

Juniperus Bibliography by Workform
Juniperus Bibliography by Workform Record Number: 2110 Author, Analytic: Abdullah-Al-Refai//El-Kateb, H//Stimm, B//Mosandl, R Author Role: Author Affiliation: Article Title: Quality and germination of seeds of Juniperus excelsa M.-Bieb. in the Kalamoun mountains, Syria. Medium Designator: Connective Phrase: Author, Monographic: Author Role: Journal Title: Forstliche Forschungsberichte Munchen Reprint Status: Date of Publication: 2003 Volume Identification: 192 Issue Identification: Page(s): Address/Availability: Lehrstuhl fur Waldbau und Forsteinrichtung, TU Munchen, Germany Location/URL: CODEN: ISSN: 0174-1810 Notes: Abstract: Juniperus excelsa is the main tree species of forest stands in the upper elevations of the Kalamoun mountains in Syria. In this preliminary experiment seeds of juniper from four stands in different elevations (1900, 2100, 2200, 2250 m) were subjected to two pre-treatments with different duration period: warm stratification for three-months followed by 45 days warm stratification followed by 45 days cold stratification, and six months with 90 days warm followed by 90 days cold stratification. In comparison to the other three stands, the stand 2100 above sea level had more vigorous trees from which the seeds were collected. After stratification, seed samples were subjected to a standard germination test according to ISTA regulations. Juniper seeds originating from the Kalamoun mountains showed with 92.5% a high percentage of empty seeds. The better quality of seeds with less empty seeds (87%) were found in the stand which included the more vigorous juniper trees in 2100 m above sea level. Germination of seeds was significantly dependant on the duration period of the warm and cold stratification. -
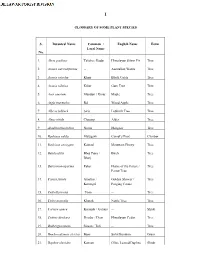
GLOSSARY of SOME PLANT SPECIES S. No. Botanical Name
I GLOSSARY OF SOME PLANT SPECIES S. Botanical Name Common / English Name Form Local Name No. 1. Abies pindrow Talisha / Badar Himalayan Silver Fir Tree 2. Acacia auriculiformis -- Australian Wattle Tree 3. Acacia catechu Khair Black Cutch Tree 4. Acacia nilotica Kikar Gum Tree Tree 5. Acer caesium Mandari / Kinar Maple Tree 6. Aegle marmelos Bel Wood Apple Tree 7. Albizia lebbeck Siris Lebback Tree Tree 8. Alnus nitida Chaamp Alder Tree 9. Azadinachta indica Neem Margosa Tree 10. Bauhinia vahlii Malugarh Camel’s Foot Climber 11. Bauhinia variegate Kakrad Mountain Ebony Tree 12. Betula utilis Bhoj Patra / Birch Tree Bharj 13. Butea monosperma Palas Flame of the Forest / Tree Parrot Tree 14. Cassia fistula Amaltas / Golden Shower / Tree Karangal Purging Cassia 15. Cedrella toona Toon -- Tree 16. Celtis australis Kharak Nettle Tree Tree 17. Carissa opaca Karonda / Garana -- Shrub 18. Cedrus deodara Deodar / Dear Himalayan Cedar Tree 19. Dalbergia sissoo Sissoo / Tali -- Tree 20. Dendrocalamus strictus Bans Solid Bamboo Grass 21. Daphne oleoides Kansan Olive Leaved Daphne Shrub II 22. Dodonaea viscose Santha Jamaica Switch Sorrel Bush 23. Emblica officinalis Amla -- Tree 24. Euphorbia royleana Thor -- Shrub 25. Fiscus bengalensis Bargad / Bod -- Tree 26. Flacourtia indica Kakoa Medagasker Plum Tree 27. Grevillea robusta -- Silver Oak Tree 28. Grewia optiva Dhamman -- Tree 29. Ilex dipyrena Khareii Himalayan Holly Tree 30. Indigofera heterantha Katai -- Shrub 31. Jacaranda mimisifolia Nili Gulmohar Mimosa Leaved Ebony Tree 32. Juglans regia Akhrot (Khod) Walnut Tree 33. Juniperus indica -- -- Tree 34. Lannea coromandelica Kembal / Jhingar -- Tree 35. Lantana camara Jadi / Panchfuli Yellow Sage Shrub 36. Lantana sillowiana Jadi / Panchfuli Weeping Lantana Shrub 37.