Phenolic Content, Chemical Composition and Anti-/Pro-Oxidant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Waldo County Soil and Water Conservation District Annual Fruit Tree and Shrub Sale
Waldo County Soil and Water Conservation District Annual Fruit Tree and Shrub Sale 2018 Offerings We are returning this year with many carefully selected fruit trees, berry plants and landscape shrubs and perennials that will enhance your property’s health, natural balance, beauty and productivity. Our Landscape Plants theme this year is Wet and Dry: Beautiful Natives for Rain Gardens, Wet Areas and Shorelines. The plants offered will also tolerate drier conditions. We are also returning with popular Inside this issue plants that offer year-round color and interest, and that are good substitutes for invasive plants now Inside Story Inside Story banned. Buttonbush, at right, is a lovely shoreline Inside Story bush that can stand inundation...and also has Inside Story beautiful flowers that feed butterflies and retain their form into the winter. Important Dates 05/31 06/26 Native shrubs such as the highbush or American cranberry (at left) offer flowers, fruits and fall color that is as 07/29 attractive as invasive shrubs like burning bush or Japanese barberry. Each landscape plant we offer has been selected for multi-season interest and color as well as wildlife value. Fruit Trees and Shrubs SEMI-DWARF APPLE On MM111 rootstock – semi dwarf trees – grow 15-20’ but can be pruned to a shorter height. Hardy and quicker to bear fruit than standard trees. 1. Wolf River Apple – (Left) Huge heirloom, great for pies, making a big comeback in Maine. Disease resistant and extremely hardy. 2. Holstein Apple – Seedling of Cox Orange Pippin, but larger. Creamy, yellowish, juicy flesh is aromatic and very flavorful. -

An Old Rose: the Apple
This is a republication of an article which first appeared in the March/April 2002 issue of Garden Compass Magazine New apple varieties never quite Rosaceae, the rose family, is vast, complex and downright confusing at times. completely overshadow the old ones because, as with roses, a variety is new only until the next This complexity has no better exemplar than the prince of the rose family, Malus, better known as the variety comes along and takes its apple. The apple is older in cultivation than the rose. It presents all the extremes in color, size, fragrance place. and plant character of its rose cousin plus an important added benefit—flavor! One can find apples to suit nearly every taste and cultural demand. Without any special care, apples grow where no roses dare. Hardy varieties like the Pippins, Pearmains, Snow, Lady and Northern Spy have been grown successfully in many different climates across the U.S. With 8,000-plus varieties worldwide and with new ones introduced annually, apple collectors in most climates are like kids in a candy store. New, Favorite and Powerhouse Apples New introductions such as Honeycrisp, Cameo and Pink Lady are adapted to a wide range of climates and are beginning to be planted in large quantities. The rich flavors of old favorites like Spitzenburg and Golden Russet Each one is a unique eating experience that are always a pleasant surprise for satisfies a modern taste—crunchy firmness, plenty inexperienced tasters. of sweetness and tantalizing flavor. Old and antique apples distinguish These new varieties show promise in the themselves with unusual skin competition for the #1 spot in the world’s colors and lingering aftertastes produce sections and farmers’ markets. -
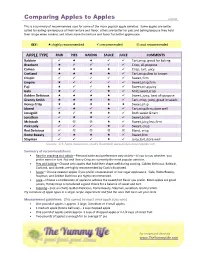
Comparing Apples to Apples 10/2010
Comparing Apples to Apples 10/2010 This is a summary of recommended uses for some of the more popular apple varieties. Some apples are better suited for eating raw because of their texture and flavor, others are better for pies and baking because they hold their shape when cooked, and others have the texture and flavor for better applesauce. KEY: =highly recommended =recommended =not recommended APPLE TYPE RAW PIES BAKING SAUCE JUICE COMMENTS Baldwin Tart,crisp, great for baking Braeburn Crisp, all-purpose Cameo Crisp, tart, juicy Cortland Tart,crisp;slow to brown Crispin Sweet, firm Empire Sweet,crisp,firm Fuji Sweet,crisp,juicy Gala Mild,sweet,crisp Golden Delicious Sweet, juicy, best all-purpose Granny Smith Tart, crisp, juicy, great in salads Honey Crisp Sweet,crisp Idared Tart,crisp,firm,store well Jonagold Both sweet & tart Jonathan Sweet,acidic McIntosh Sweet,juicy,less firm Pink Lady Sweet, crisp Red Delicious Bland, crisp Rome Beauty Sweet,firm Stayman Juicy,tart,store well Sources: U.S. Apple Association, Cook’s Illustrated, www.pickyourown/apples.com Summary of recommendations: • Raw for snacking and salads —Personal taste and preferences vary widely—it’s up to you whether you prefer sweet or tart. Fuji and Honey Crisp are currently the most popular varieties. • Pies and baking —Choose only apples that hold their shape well during cooking. Golden Delicious, Baldwin, Cortland, and Idareds are highly recommended by Cook’s Illustrated. • Sauce —Choose sweeter apples if you prefer unsweetened or low-sugar applesauce. Gala, Rome Beauty, Stayman, and Golden Delicious are highly recommended. • Juice —Choose a combination of apples to achieve the sweet/tart flavor you prefer. -

Inaugural Issue
THE ORTET Inaugural Issue Midwest Apple Improvement Association Autumn 2015 Newsletter MAIA Board of Directors IN THIS ISSUE Gregg Bachman Sunny Hill Fruit Farm, Carroll, OH 3 A Message from the Chairman Felix Cooper Garden’s Alive!, Tipp City, OH 4 President’s Report David Doud Countyline Orchard, Wabash, IN 5 MAIA Apple Evaluation iPad App Jim Eckert Eckert Orchards, Belleville, IL 6 An Embarrassment of Riches Allen Grobe Grobe Fruit Farm, Elyria, OH 7 An Article from the Archives David Hull White House Fruit Farm, Canfield, OH 8 Fall Taste Evaluations Dano Simmons Peace Valley Orchard, Rogers, OH 13 Apple Crunch Day Andy Lynd Lynd Fruit Farm, Pataskala, OH 14 Apples for New Era MAIA Advisors Mitch Lynd Lynd Fruit Farm, Pataskala, OH Dr. Diane Miller The Ohio State University Bill Dodd Hillcrest Orchard, Amherst, OH Newsletter Staff, Ciderwood Press Amy Miller, Editor-in-Chief Midwest Apple Improvement Association Matt Thomas, Creative Director P.O. Box 70 Kathryn Everson, Layout Editor Newcomerston, OH 43832 (800) 446 - 5171 To obtain the EverCrisp® licens- To find out how to become a member, learn ing agreement and learn more about more about our history, read past newsletters, MAIA1 go to: and more, go to: evercrispapple.com midwestapple.com 2 A Message from the Chairman Greetings All, ometimes we find value in unexpected places. An example S of this at our farm market is a used Pease apple peeler we bought several years ago to make peeling more efficient in our bakery. After a few weeks of using the peeler, the girls found that they liked their smaller household peelers better than using the bigger, although more efficient, peeler. -

Brightonwoods Orchard
Managing Diversity Jimmy Thelen Orchard Manager at Brightonwoods Orchard 2020 Practical Farmers of Iowa Presentation MAP ORCHARD PEOPLE ORCHARD PEOPLE • UW-Parkside Graduate • Started at Brightonwoods in 2006 • Orchard Manager and in charge of Cider House • Case Tractor Hobby & Old Abe's News ORCHARD HISTORY • Initial sales all from on the farm (1950- 2001) “Hobby Orchard” • Expansion into multiple cultivars (10 acres) • 1980's • Added refrigeration • Sales building constructed ORCHARD HISTORY • Retirement begets new horizons • (1997-2020) • Winery (2000-2003) additional 2 acres of trees for the winery and 30+ varieties of apples & pears ORCHARD HISTORY • Cider House (2006) with UV light treatment and contract pressing • Additional ½ acre of Honeycrisp ORCHARD HISTORY • Additional 3 acres mixed variety higher density planting ~600 trees per acre ORCHARD HISTORY • Addition of 1 acre of River Belle and Pazazz ORCHARD • Not a Pick- your-own • All prepicked and sorted • Not Agri- entertainment focused ACTIVITIES WHERE WE SELL • Retail Focused • At the Orchard • Summer / Fall Farmers' Markets • Winter Farmers' Markets • Restaurants • Special Events ADDITIONAL PRODUCTS • Honey, jams & jellies • Pumpkins & Gourds • Squash & Garlic • Organic vegetables on Sundays • Winery Products • Weekend snacks and lunches 200+ VARIETIES Hubardtson Nonesuch (October) Rambo (September) Americus Crab (July / August) Ida Red (October) Red Astrashan (July–August) Arkansas Black (October) Jersey Mac (July–August) Red Cortland(September) Ashmead's Kernal (October) -

Exile for Dreamers - Tess’S Story Kathleen Baldwin Baldwin, Page 1
Exile for Dreamers - Tess’s Story Kathleen Baldwin Baldwin, page 1 Exile for Dreamers —A Stranje House Novel— By Kathleen Baldwin Exile for Dreamers - Tess’s Story Kathleen Baldwin Baldwin, page 2 Dedication To the men who taught me to run, swim, hunt, gallop horses, ride the rapids, ski powder, hang-glide, and rock climb - back when girls did not do that sort of thing. To Daddy, for teaching me to box at a time when it was sacrilegious to strap a pair of boxing gloves on a little girl, and to the boys whose noses I broke – I am truly sorry. And to Susan Thank you for your extraordinary skills as an editor. I’m grateful you can see when I am blind. Exile for Dreamers - Tess’s Story Kathleen Baldwin Baldwin, page 3 Chapter 1 Tess I run to escape my dreams. Dreams are my curse. Every night they haunt me, every morning I outrun them, and every evening they catch me again. One day they will devour my soul. But not today. Not this hour. I ran with Phobos and Trobos, the half wolves half dogs who guard Stranje House. We raced into the cleansing wind. What is the pace of forgetfulness? How fast must one go? “Tess! Wait!” Georgiana’s gasps cut through the peace of the predawn air and broke my rhythm. I slowed to a stop and turned. A moment later, Phobos broke stride too, and trotted back beside me. He issued a low almost imperceptible growl, impatient to return to our race. Georgie leaned forward breathing hard. -

Americus Crab Americus Crab -Side Arkansas Black Arkansas Black -Side Ashmeads Kernel
Americus Crab Americus Crab -side Arkansas Black Arkansas Black -side Ashmeads Kernel Ashmeads Kernel -side Baldwin Baldwin -side Beacon Beacon -side Bella Vista Bella Vista -side Binet Blanc Binet Blanc -side Blenheim Orange Blenheim Orange -side Blushing Golden Blushing Golden -side BWO Early WI Red BWO Early WI Red -side BWO Sweet Thingy BWO Sweet Thingy -side Calville Blanc dHiver Calville Blanc dHiver -side Chenango Strawberry Chenango Strawberry -side Chestnut Crab Chestnut Crab -side Chisel Jersey (faux) -side Chisel Jersey (faux) Coat Jersey Coat Jersey -side Connell Red Connell Red -side Coos Bay Late Coos Bay Late -side Cornell Sour Cornell Sour -side Cornell Sweet Cornell Sweet -side Cortland Cortland -side Cox's Orange Pippin Cox's Orange Pippin -side Cricket Creek Yellow Crab Cricket Creek Yellow Crab - Crimson King Crimson King -side Crispin Crispin -side side Davey Davey -side Dolgo Crab Dolgo Crab -side Domaine Domaine -side DS-99 DS-99 -side DS 428 DS 428 -side Duchess of Oldenburg Duchess of Oldenburg -side Early Jon Early Jon -side Empire Empire -side English Golden Russet English Golden Russet -side Esopus Spitzenberg Esopus Spitzenberg -side Fireside Fireside - side Frequin Rouge Frequin Rouge -side Fuji Fuji -side Gala Gala -side Gallia Beauty Gallia Beauty -side Genesis II Genesis II -side Golden Delicious Golden Delicious -side Golden Nugget Golden Nugget -side Granny Smith Granny Smith -side Gravenstein Gravenstein -side Greendale Greendale -side Grimes Golden Grimes Golden -side Haralson Haralson -side Holdren's -

2019 ANNUAL REPORT a National Leader in Animal Care, Conservation and Visitor Experience
Providing a level of excellence that makes the Rosamond Gifford Zoo 2019 ANNUAL REPORT a national leader in animal care, conservation and visitor experience. 1 A JOINT MESSAGE FROM THE INTERIM EXECUTIVE DIRECTOR AND THE CHAIR OF THE BOARD There is much to celebrate in the Friends 2019 annual report. Last year saw the birth of a baby Asian elephant and two critically endangered Amur leopard cubs. The zoo unveiled several new exhibits and welcomed several new rare and endangered species. Friends of the Zoo and our county partners upgraded and expanded the Helga Beck Asian Elephant Preserve, and construction began on a key Friends project, the Zalie & Bob Linn Amur Leopard Woodland. Some 332,000 people visited the zoo, enhanced by the Friends’ addition of a summer-long Big Bugs! attraction and a new 18-horse carousel. Our education programs involved more than 10,000 participants, including more than 7,500 school-age children. Our volunteers provided educational enrichment to guests, beautified the zoo with lush gardens and joined work projects to improve the facility. Our catering staff executed more than 120 events, from weddings to the Snow Leopard Soirée to Brew at the Zoo. All told, Friends provided more than $175,000 in direct support to the zoo and allocated more than $500,000 for capital improvements to help the zoo meet the “gold standard” for animal care and welfare, conservation education and saving species. We are extremely proud of these accomplishments, and we hope you will enjoy this look back at our achievements and all the people, businesses and community partners that helped make them possible. -

The Church Family Orchard of the Watervliet Shaker Community
The Church Family Orchard of the Watervliet Shaker Community Elizabeth Shaver Illustrations by Elizabeth Lee PUBLISHED BY THE SHAKER HERITAGE SOCIETY 25 MEETING HOUSE ROAD ALBANY, N. Y. 12211 www.shakerheritage.org MARCH, 1986 UPDATED APRIL, 2020 A is For Apple 3 Preface to 2020 Edition Just south of the Albany International called Watervliet, in 1776. Having fled Airport, Heritage Lane bends as it turns from persecution for their religious beliefs from Ann Lee Pond and continues past an and practices, the small group in Albany old cemetery. Between the pond and the established the first of what would cemetery is an area of trees, and a glance eventually be a network of 22 communities reveals that they are distinct from those in the Northeast and Midwest United growing in a natural, haphazard fashion in States. The Believers, as they called the nearby Nature Preserve. Evenly spaced themselves, had broken away from the in rows that are still visible, these are apple Quakers in Manchester, England in the trees. They are the remains of an orchard 1750s. They had radical ideas for the time: planted well over 200 years ago. the equality of men and women and of all races, adherence to pacifism, a belief that Both the pond, which once served as a mill celibacy was the only way to achieve a pure pond, and this orchard were created and life and salvation, the confession of sins, a tended by the people who now rest in the devotion to work and collaboration as a adjacent cemetery, which dates from 1785. -

Postal Service Issues Apples Postcard Stamps
FOR IMMEDIATE RELEASE Contact: Mark Saunders Jan. 17, 2013 [email protected] 202.268.6524 usps.com/news Release No. 13-004 Postal Service Issues Apples Postcard Stamps High-resolution images of the stamps are available for media use only by emailing [email protected]. YAKIMA, WA — Postcards navigating the nation’s mail stream will begin to bear four varieties of fruit now that the Postal Service has harvested the 33-cent Apples Postcard stamps. Available today in panes of 20 or coils of 100, customers may purchase the stamps now at usps.com/stamps, by phone at 800-STAMP24 (800-782-6724) and at Post Offices nationwide to prepare for the Jan. 27 1-cent price change. Featuring (clockwise from top left) images of the Northern Spy, Golden Delicious, Baldwin and Granny Smith apples, the stamp art was illustrated with pen and ink and watercolor, with some additional detail added on computer. Designed by art director Derry Noyes of Washington, DC, the Apples Postcard stamps features the work of John Burgoyne of West Barnstable, MA. 2 Northern Spy “Spies are for pies!” The homey little rhyme offers a reminder that generations of cooks have found the Northern Spy apple delicious when baked in desserts. Its tart, tangy taste makes it less of a favorite for eating in hand, for some people, but it stores well and tends to last longer because of its late season. This variety is also good for cider and juice. A Northern Spy apple tree was reportedly “discovered” around 1800 near Rochester, NY. -

Plant Disease
report on RPD No. 816 PLANT October 1994 DEPARTMENT OF CROP SCIENCES DISEASE UNIVERSITY OF ILLINOIS AT URBANA-CHAMPAIGN POWDERY MILDEW OF APPLE Powdery mildew, caused by the fungus Podosphaera leucotricha, occurs wherever apples are grown. Other susceptible plants include crabapple, pear, quince, and photinia. Powdery mildew was a very minor disease in Midwest apple orchards for many years. The principal fungicide then used was sulfur. As organic fungicides including captan, dodine, and zineb came into general use and as sulfur was gradually eliminated from spray programs, powdery mildew became much more prevalent. The disease is common in Illinois south of a line from Quincy to Figure 1. Powdery Mildew on young apple leaves. Mattoon and becoming a more serious problem in orchards north of that line also. Of all apples grown in Illinois, the Jonathan is probably the variety most susceptible to mildew. Other susceptible varieties include Baldwin, Corland, Golden Delicious, Grimes Golden, Idared, Monroe, Rome, and Stayman. Mildew is usually less common on McIntosh, Prima, Priscilla, Red Delicious, and Winesap, however, no apple variety is immune to powdery mildew. Damage occurs on the leaves, twigs, blossoms, and fruit: 1. Leaves and twig terminals are stunted, deformed, or killed. 2. Blossoms are blighted, so the fruit may fail to set. 3. The fruit that does mature is disfigured by surface russeting. 4. Fewer blossom buds develop for the following year. If left uncontrolled, powdery mildew becomes more serious each year, reducing the yield and the quality of the fruit. SYMPTOMS The powdery mildew fungus infects the lateral and terminal buds, young leaves, twigs, blossoms, and green fruits. -

Growing Apples for Craft Ciders Ian A
Growing Apples for Craft Ciders Ian A. Merwin Professor of Horticulture Emeritus—Cornell University Grower and Cider-maker—Black Diamond Farm ider has been a mainstay food and fermented bever- ies”) around the country are now seeking apple varieties known age for thousands of years. Domesticated apples were for making top quality ciders. The demand for these varieties brought to America by the first European colonists, and greatly exceeds their current supply, because only a few of the C from 1640 to 1840 new cideries have productive orchards or expertise in growing “There is significant growing interest in most of our or- apples. The un-met demand for special cider apples has led chards consisted growers across the US and Canada to consider these apples as hard cider with the number of cideries of seedling apples, an alternative to growing mainstream varieties, because the best- in NY State reaching 53. In this article grown primar- known cider apples fetch prices as high as $400 per 20-bushel I summarize our 30 years of experience ily for sweet and bin. My purpose in writing this article is to summarize what I at Cornell and on my own farm of hard (fermented) have learned about growing these cider apples over the past 30 growing hard cider varieties.” cider. Despite years, and to make this information available to those interested this long history, in cider and cider apples. hard cider was not considered A Note of Caution an economically important drink in the US until quite recently, Modern orchards cost about $25,000 per acre to establish and when the USDA and several apple-growing states began to col- bring them into commercial production.