Johnson & Johnson Vision Fast Facts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MRSA Ophthalmic Infection, Part 2: Focus on Orbital Cellulitis
Clinical Update COMPREHENSIVE MRSA Ophthalmic Infection, Part 2: Focus on Orbital Cellulitis by gabrielle weiner, contributing writer interviewing preston h. blomquist, md, vikram d. durairaj, md, and david g. hwang, md rbital cellulitis is a poten- Acute MRSA Cellulitis tially sight- and life-threat- ening disease that tops the 1A 1B ophthalmology worry list. Add methicillin-resistant OStaphylococcus aureus (MRSA) to the mix of potential causative bacteria, and the level of concern rises even higher. MRSA has become a relatively prevalent cause of ophthalmic infec- tions; for example, one study showed that 89 percent of preseptal cellulitis S. aureus isolates are MRSA.1 And (1A) This 19-month-old boy presented with left periorbital edema and erythema preseptal cellulitis can rapidly develop five days after having been diagnosed in an ER with conjunctivitis and treated into the more worrisome condition of with oral and topical antibiotics. (1B) Axial CT image of the orbits with contrast orbital cellulitis if not treated promptly shows lacrimal gland abscess and globe displacement. and effectively. Moreover, the community-associ- and Hospital System in Dallas, 86 per- When to Suspect ated form of MRSA (CA-MRSA) now cent of those with preseptal cellulitis MRSA Orbital Cellulitis accounts for a larger proportion of and/or lid abscesses had CA-MRSA. Patients with orbital cellulitis com- ophthalmic cases than health care– These studies also found that preseptal monly complain of pain when moving associated MRSA (HA-MRSA). Thus, cellulitis was the most common oph- the eye, decreased vision, and limited many patients do not have the risk fac- thalmic MRSA presentation from 2000 eye movement. -

Adult Patients Common Eye Infections
Common Eye Dermatitis: HZV and HSV Infections: Adult • Redness of periocular skin can be allergic Patients (if associated with prominent itching) or bacterial (if associated with open sores/wounds) Julie D. Meier, MD Assistant Professor of Ophthalmology • Both HZV and HSV can have devastating ocular sequelae if not treated promptly OSU Eye and Ear Institute General Categories of Herpes Zoster Eye Infections Ophthalmicus • Symptoms: Skin rash and pain, may be • Dermatitis of Lids (HZV, HSV) preceded by headache, fever, eye pain or • Cellulitis of Lids (pre- vs post-septal) blurred vision • Blepharitis • Signs: Vesicular skin rash involving CN V • Conjunctivitis distribution; Involvement of tip of nose can predict higher rate of ocular involvement • Keratitis 1 Herpes Zoster Herpes Simplex Virus Ophthalmicus • Symptoms: • Work-up 9 Duration of rash; Immunocompromised? 9 Red eye, pain, light sensitivity, skin rash 9 Complete ocular exam, including slit 9 Fever, flu-like symptoms lamp, IOP, and dilated exam • Signs: • Can have conjunctival or corneal involvement, elevated IOP, anterior 9 Skin rash: Clear vesicles on chamber inflammation, scleritis, or erythematous base that progress to even involvement of retina and optic crusting nerve. Herpes Zoster Herpes Simplex Virus Ophthalmicus • Work-up: • Treatment: 9 Previous episodes? 9 If present within 3 days of rash’s 9 Previous nasal, oral or genital sores? appearance: oral Acyclovir/ Valacyclovir 9 Recurrences can be triggered by fever, stress, trauma, UV exposure 9 Bacitracin ointment to skin lesions 9 External exam: More suggestive of HSV 9 Warm compresses if lesions centered around eye and no involvement of forehead/scalp 9 TOPICAL ANTIVIRALS (e.g. -

Common Eye Condition Management
Common eye condition management Introduction by Moorfields’ medical director Thank you for taking the time to read this concise advice booklet about common eye conditions. It has been produced by clinicians and other staff CONTENTS at Moorfields to help you to make informed clinical decisions about your Introduction by Moorfields’ patients’ eye conditions locally, and medical director ......................... 3 avoid them having to attend hospital unnecessarily. Schematic diagram of the human eye ........................ 4 For each of the most common conditions you might see in your practice, we have listed signs and symptoms, General information Equipment and drugs to keep the equipment you will need to examine the patient, and at hand in the surgery ............ 4 the procedure to follow in undertaking that examination. General good practice advice ..................................... 5 Towards the end of the booklet, we have included a Eye examination .................... 5 table divided into four levels of urgency for onward referral – immediate, within 24 hours, within one week Care pathways for common and routine – with a list of relevant circumstances and eye conditions: conditions for each. Conjuntivitis ........................... 6 Dry eyes ............................... 7 We have also provided a table of the several locations Blepharitis ............................. 8 in which Moorfields provides care in and around Chalazion (meibomian cyst) ...10 London, and the sub-specialty services we offer in Stye .......................................11 each place. Corneal abrasion ....................12 Corneal foreign body ..............13 Subtarsal foreign body ..........14 I hope you find this guide helpful, and welcome your Subconjunctival views on how we might improve future editions. Please haemorrhage .........................15 contact our GP liaison manager on 020 7253 3411, Episcleritis .............................16 ext 3101 or by email to [email protected] with your comments. -

STYES and CHALAZION
TRE ATM ENT TRE ATM ENT FOR STYES FOR CHALAZION While most styes will drain on their The primary treatment for chalazion is own, the application of a hot or warm application of warm compresses for 10 compress are the most effective to 20 minutes at least 4 times a day. means of accelerating This may soften the hardened oils STYES drainage. The blocking the duct and promote drain- warmth and damp- age and healing. ness encourages the stye to drain. Just like any infection try not to touch it with your fingers. A Chalazion may be treated with compress can be made by putting hot any one or a combination of (not boiling) water on a wash cloth, or antibiotic or steroid drops pre- by using room temperature water and scribed by your healthcare a plastic heat pack. Warm compress- provider. es should be applied for 10—20 and minutes, four (4) times a day. There are occasions when sur- There is also a specialized topical gical drainage is required. ointment for styes, that may be pre- scribed. “Do not use eye makeup Styes may also cause a bruised feel- or wear contact lenses ing around the eye which is treated by application of a warm cloth to the eye. until the stye or chalazion CHALAZION With treatment, styes typically resolve have healed.” within one week. Lancing of a stye is not recommended. Revised: August 2011 WHAT ARE THEY? Signs and Symptoms Signs & Symptoms O f S t ye s of Chalazions The first signs of a stye are: A stye is an infection of the The symptoms of chalazions differ from tenderness, sebaceous glands at the base of the styes as they are usually painless. -

Eye Infections
CLINICAL Approach Taking a Look at Common Eye Infections John T. Huang, MD, FRCSC and Peter T. Huang, MD, FRCSC he acutely red eye is often seen first by the primary-care physician. The exact Tcause may be difficult to determine and may cause some concern that a serious ocular condition has been missed. Thorough history and clinical examination will help delineate the final diagnosis. When there are doubts, prompt referral to an oph- thalmologist can prevent serious consequences. Often, the most likely diagnosis of an acutely red eye is acute conjunctivitis. In the first day, an acute bacterial infection may be hard to differentiate from viral, chlamydial and noninfectious conjunctivitis and from episcleritis or scleritis. Below is a review of the most commonly seen forms of eye infections and treat- ments. Failure to improve after three to five days should lead to a re-evaluation of the patient and appropriate referral where necessary. CHRONIC BLEPHARITIS Clinical: Gritty burning sensation, mattering, lid margin swelling and/or scaly, flaky debris, mild hyperemia of conjunctiva; may have acne rosacea or hyperkeratotic dermatitis (Figure 1). Anterior: Staphylococcus aureus (follicles, accessory glands); posterior (meibomian glands). Treatment: • Lid scrubs (baby shampoo, lid-care towellettes, warm compresses). Figure 1. Chronic blepharitis. There may be localized sensitivity to the shampoo or the components of the solution in the towellettes (e.g., benzyl alcohol). • Hygiene is important for the treatment and management of chronic blepharitis. Topical antibiotic-corticosteroid combinations (e.g., tobramycin drops, tobramycin/dexamethasone or sulfacetamide sodium-prednisolone acetate). Usage of these medications is effective in providing symptomatic relief, as the inflammatory component of the problem is more effectively dealt with. -

Stye (Hordeolum) N
n Stye (Hordeolum) n What puts your child at risk A stye is an infection causing a red, swollen bump on the eyelid. It occurs when the glands of a stye? under the skin of the eyelid become infected. Anything that irritates the eye, including frequent rub- Treatment, possibly including antibiotics, is impor- bing, eye makeup, or contact lenses, may increase the tant to prevent the infection from spreading. risk of infection. However, most styes occur without such risk factors. Other infections of the eyelid (such as blepharitis) may What is a stye? increase the risk of styes. A stye is an infection of the glands under the skin of the Can styes be prevented? eyelid, at the base of the eyelashes. The medical term is “hordeolum.” Styes can be quite irritating, and there is a Good hygiene, including regular washing of the face and risk that the infection will spread. hands, may reduce the risk of styes. Treatment usually consists of frequent soaks with a warm washcloth. Your doctor may recommend an antibiotic oint- ment as well. If the stye doesn’t go away within a few days, How are styes treated? or if it seems to be getting worse, call our office. Warm soaks. Soak a washcloth in warm water and place it over the eye. Keep the warm washcloth on the eye for 10 minutes or so, a few times per day. This will reduce What does it look like? pain and help the stye to heal faster. A red, tender, swollen bump on the edge of the eyelid. -

The Eyes Have It! Update on Common Conditions Affecting the Eye
Pharmacy Tech Topics™ VOLUME 22 NO. 2 | APRIL 2017 The Eyes Have It! Update on Common Conditions Affecting the Eye AUTHORS: Steven R. Abel, BS Pharm, PharmD, FASHP Kirk Evoy, PharmD, BCACP, BC-ADM, TTS PEER REVIEWERS: Sami Labib, RPh Rita Edwards, CPhT EDITOR: Patricia M. Wegner, BS Pharm, PharmD, FASHP DESIGN EDITOR: Leann Nelson Pharmacy Tech Topics™ (USPS No. 014-766) is published quarterly for $50 per year by the Illinois Council of Health-System Pharmacists, 4055 N. Perryville Road, Loves Park, IL 61111-8653. Phone 815-227-9292. Periodicals Postage Paid at Rockford, IL and additional mailing offices. POSTMASTER: Send address changes to: Pharmacy Tech Topics™, c/o ICHP, 4055 N. Perryville Road, Loves Park, IL 61111-8653 COPYRIGHT ©2017 by the Illinois Council of Health-System Pharmacists unless otherwise noted. All rights reserved. Pharmacy Tech Topics™ is a trademark of the Illinois Council of Health-System Pharmacists. This module is accredited for 2.5 contact hours of continuing pharmacy education and is recognized by the Pharmacy Technician Certification Board (PTCB). Cover image property of ©2017 Adobe Stock. LEARNING OBJECTIVES Upon completion of this module, the subscriber will be able to: 1. Identify the parts of the eye and the function of each part. 2. Summarize various eye disorders including ocular hypertension, glaucoma, infections, dry eyes, conjunctivitis, age-related macular degeneration, macular edema following retinal vein occlusion, and diabetic macular edema. 3. Discuss brand/generic substitutions, possible side effects, and proper administration of ophthalmic medications. 4. Describe the roles of various ophthalmic agents including those used to treat the following conditions: ocular hypertension, glaucoma, infections, dry eyes, conjunctivitis, age-related macular degeneration, macular edema following retinal vein occlusion, and diabetic macular edema. -
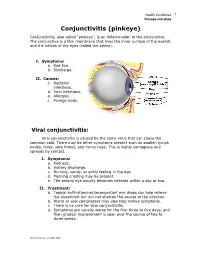
Pinkeye and Styes Conjunctivitis (Pinkeye) Conjunctivitis, Also Called “Pinkeye”, Is an Inflammation of the Conjunctiva
Health Guidelines 1 Pinkeye and styes Conjunctivitis (pinkeye) Conjunctivitis, also called “pinkeye”, is an inflammation of the conjunctiva. The conjunctiva is a thin membrane that lines the inner surface of the eyelids and the whites of the eyes (called the sclera). I. Symptoms: a. Red Eye. b. Discharge. II. Causes: c. Bacterial infections. d. Viral infections. e. Allergies. f. Foreign body. Viral conjunctivitis: Viral conjunctivitis is caused by the same virus that can cause the common cold. There may be other symptoms present such as swollen lymph nodes, fever, sore throat, and runny nose. This is highly contagious and spreads by contact. I. Symptoms: a. Redness. b. Watery discharge. c. Burning, sandy, or gritty feeling in the eye. d. Morning crusting may be present. e. The second eye usually becomes infected within a day or two. II. Treatment: a. Topical antihistamine/decongestant eye drops can help relieve the discomfort but will not shorten the course of the infection. b. Warm or cool compresses may also help relieve symptoms. c. There is no cure for viral conjunctivitis. d. Symptoms are usually worse for the first three to five days, and then gradual improvement is seen over the course of two to three weeks. 2013, 8-28 JJustad, MD, DDP Health Guidelines 2 Pinkeye and styes Bacterial conjunctivitis: Bacterial conjunctivitis is highly contagious and spread by contact. I. Symptoms: a. Redness and thick discharge from one eye. b. Both eyes can become infected. c. The discharge is usually yellow, white, or green. d. There is discharge throughout the day. e. The eye may be “stuck shut” in the mornings. -

Specialist Clinic Referral Guidelines OPHTHALMOLOGY
Specialist Clinic Referral Guidelines OPHTHALMOLOGY COVID-19 Impact — Specialist Clinics As part of Alfred Health’s COVID-19 response plan, from October significant changes have been made to Specialist Clinic (Outpatient) services. All referrals received will be triaged; however, if your patient’s care is assessed as not requiring an appointment within the next three months, the referral may be declined. Where possible, care will be delivered via telehealth (phone or video consultation). Please fax your referral to The Alfred Specialist Clinics on 9076 6938. The Alfred Outpatient Referral Form is available to print and fax. Where appropriate and available, the referral may be directed to an alternative specialist clinic or service. You will be notified when your referral is received. Your referral may be declined if it does not contain essential information required for triage, or if the condition is not appropriate for referral to a public hospital, or is a condition not routinely seen at Alfred Health. The clinical information provided in your referral will determine the triage category. The triage category will affect the timeframe in which the patient is offered an appointment. Referral to Victorian public hospitals is not appropriate for: Review or treatment of neovascular (wet) age-related macular degeneration (AMD) where the patient has already commenced treatment at another facility Early intermediate or geographic atrophy (dry) age-related macular degeneration. If the patient is not willing to have surgical treatment Cataract that does not have a significant impact on the person’s activities of daily living Prior to the person’s vision being corrected with spectacles, contact lenses, or the use of visual aids. -

Every Red Eye Deserves an Antibiotic
Ocular Infection Management- The Next Generation Bruce E. Onofrey, OD, RPh, FAAO Professor, U. Houston UEI SERIOUS PROBLEMS REQUIRE SERIOUS DOCUMENTATION THE BIG 6 “I’s” • INFECTION • INFLAMMATION • ISCHEMIA • INJURY • IDIOPATHIC • IATROGENIC (idiotpathic) Just the facts Mrs. Johnson • 65 y/o female visiting her friend Madge from Portland, Oregon. • Madge is one of your favorite long-term patients • Madge refers patient to you • Patient is very concerned about her “eye infection” The Simple Conjunctivitis Case • 65 y/o female recently in LA to visit son • Both developed red eyes • Son told mom he has genital herpes • and chlamydia • Mother seen by local ophthalmologist HX • My vision is getting worse in my left eye • It feels very “sore” and itches a lot • It waters all the time and it feels like my eye is too big for the eye socket • It’s glued shut every morning and very swollen • People are afraid to talk to me –they think I’m going to give them “pink eye” • Am I contagious? Will I lose my vision? Case : cont’d • Mom has Hx of trachoma as child and TB in remission. Worked in a TB ward-Was treated years ago • Mom wears mono-vision CL on OS only. Disposable-wears X wears X 2 weeks. Last worn 9 days ago Case : Cont’d • Eye now very painful and vision very bad • Calif. Dr said the cornea was all “torn up” • The doctor said the drops he gave me would make it better right-away-it made it worse and I stopped it after a day • I’ve been using the new drops daily and taking the pills, but it’s just getting worse every day • Am I going blind?!? QUESTION: Differential DX • 1. -

COMMON EYE COMPLAINTS July 15, 2004 Vatinee Bunya
4/24/2018 They have a lazy eye… Be Specific!! Esotropia vs. Pseudoesotropia Eyes crossing (esotropia) Eyes drifting (exotropia) Head turn Droopy eyelid Vision concerns 1 4/24/2018 www.aapos.org/terms/conditions/49 Vertical strabismus Ocular torticollis Nystagmus Finding their null point Strabismus Fusion or less strain Ptosis Chin up to see below lids Refractive Error Squinting equivalent Amblyopia Amblyopia Three main reasons for amblyopia Refractive Greater than 2 lines difference in visual ○ high myopia/hyperopia or acuity or obvious preference for fixation in anisometropia non-verbal Strabismic Induced tropia test ○ Esotropia or exotropia or hypertropia ○ Take 12 pd base down over both eyes Deprevational ○ Symmetric response= no preference ○ Cataract, corneal opacity, vitreous ○ Asymmetric response= amblyopia hemorrhage, ptosis, hemangioma 2 4/24/2018 Their eyelid is swollen… Amblyopia Treatment Force brain to use weaker eye Fix underlying etiology (give glasses, fix strab remove cataract,etc) Patch Atropine Occluding CL Fog glasses No-No arm braces Super glue Management Stye/Chalazion Stye vs. Chalazion Warm compresses Lid hygiene Erythromycin vs. Maxitrol/Tobradex Surgical excision Cellulitis Can they open their eyelids on their own? Preseptal vs. Can you get the eyelids open? Postseptal Cellulitiss 3 4/24/2018 Treatment Orbital cellulitis Results from Antiobiotics Local resistance patterns Spread of contiguous sinus disease (most common) ○ 75-85% of cases are chronic sinusitis (acute 0.5-3%) Check blood cultures first ○ Most commonly ethmoid aircells To drain or not to drain? Traumatic violation of the orbit (implantation of Worrisome optic neuropathy foreign bodies) signs Trans-septal spread of preseptal cellulitis Abscess within orbit Metastatic hematogenous spread to orbit ○ not subperiosteal ○ Valveless orbital veins Treatment failures Dental abscess to orbit Orbital cellulitis My child’s eye is red… Common organisms Staphylcoccus Aureus Streptococcus species Anaerobic If <4 years old consider H. -

Stye and Chalazion
You should see a doctor if you are uncertain what is causing your symptoms and the symptoms do Stye and Treatment for allergic not settle within a few days. Also, see a doctor urgently if any of the Chalazion eye disease following occur: Patient education leaflet General measures Symptoms change (for Try not to rub your eyes, example, light starts to hurt as this can increase the your eyes). irritation. You have pain in the eye Bathing the eyes with a flannel (mild soreness rather than soaked in cold water or with an pain is usual with over-the-counter 'eye bath' may conjunctivitis). ease symptoms. Your vision is reduced. Avoid the cause of the The eye becomes very red - in allergy, if possible. For example, particular, if it is on one side only close windows, drive with windows shut and internal air circulation on in your car, and by wear wrap-around sunglasses when out, Use glasses while riding a two wheeler. Contact Us: Change of environment- some Sanjeevani Eye Hospital people are more comfortable 1st floor, Vrindavan, away from present environment. Salasar CHS, Shifting out, usually to drier environment, helps in some Opp. Maxus Mall, extreme cases. Bhayendar West Eye drops that reduce the allergic Thane- 401101 reaction are often prescribed. Tel: 022-28162222/ 28172222 Tablets may also be prescribed, +91 9004672732 and injections have been used in e-mail- the past. [email protected] Are there any possible Eye drops usually work well. complications? You need to use them regularly to keep symptoms away until Allergic conjunctivitis can be unpleasant, but complications are the cause of the allergy goes.