Patterns of Floral Structure and Orientation in Japonolirion , Narthecium, and Tofieldia Margarita V
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Natural Heritage Program List of Rare Plant Species of North Carolina 2016
Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Revised February 24, 2017 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org C ur Alleghany rit Ashe Northampton Gates C uc Surry am k Stokes P d Rockingham Caswell Person Vance Warren a e P s n Hertford e qu Chowan r Granville q ot ui a Mountains Watauga Halifax m nk an Wilkes Yadkin s Mitchell Avery Forsyth Orange Guilford Franklin Bertie Alamance Durham Nash Yancey Alexander Madison Caldwell Davie Edgecombe Washington Tyrrell Iredell Martin Dare Burke Davidson Wake McDowell Randolph Chatham Wilson Buncombe Catawba Rowan Beaufort Haywood Pitt Swain Hyde Lee Lincoln Greene Rutherford Johnston Graham Henderson Jackson Cabarrus Montgomery Harnett Cleveland Wayne Polk Gaston Stanly Cherokee Macon Transylvania Lenoir Mecklenburg Moore Clay Pamlico Hoke Union d Cumberland Jones Anson on Sampson hm Duplin ic Craven Piedmont R nd tla Onslow Carteret co S Robeson Bladen Pender Sandhills Columbus New Hanover Tidewater Coastal Plain Brunswick THE COUNTIES AND PHYSIOGRAPHIC PROVINCES OF NORTH CAROLINA Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org This list is dynamic and is revised frequently as new data become available. New species are added to the list, and others are dropped from the list as appropriate. -
1Lecture Notes 2013
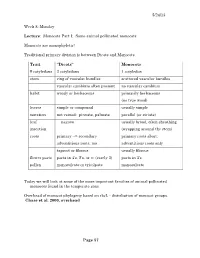
5/24/13 Week 8; Monday Lecture: Monocots Part I: Some animal pollinated monocots Monocots are monophyletic! Traditional primary division is between Dicots and Monocots Trait “Dicots” Monocots # cotyledons 2 cotyledons 1 cotyledon stem ring of vascular bundles scattered vascular bundles vascular cambium often present no vascular cambium habit woody or herbaceous primarily herbaceous (no true wood) leaves simple or compound usually simple venation net veined: pinnate, palmate parallel (or striate) leaf narrow usually broad, often sheathing insertion (wrapping around the stem) roots primary --> secondary primary roots abort; adventitious roots, too adventitious roots only taproot or fibrous usually fibrous flower parts parts in 4’s, 5’s, or ∞ (rarely 3) parts in 3’s pollen monosulcate or tricolpate monosulcate Today we will look at some of the more important families of animal pollinated monocots found in the temperate zone Overhead of monocot phylogeny based on rbcL - distribution of monocot groups. Chase et al. 2000, overhead Page 57 5/24/13 Lab only; limited discussion here. Show: “Plants are Cool, Too” video Araceae - Arum family (109 gen/2830 spp) 1) herbs (some epiphytes) 2) lvs simple or compound; broad and having an apparent petiole (‘pseudo-lamina’) development not same as in a dicot leaf blade 3) calcium oxalate crystals usually present – physical deterrent to herbivory 4) Inflorescence consisting of - spathe - bract (often colorful) surrounding the flowers - spadix - axis on which the flowers are borne (male above; female below, -

Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE
Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE LILIACEAE de Jussieu 1789 (Lily Family) (also see AGAVACEAE, ALLIACEAE, ALSTROEMERIACEAE, AMARYLLIDACEAE, ASPARAGACEAE, COLCHICACEAE, HEMEROCALLIDACEAE, HOSTACEAE, HYACINTHACEAE, HYPOXIDACEAE, MELANTHIACEAE, NARTHECIACEAE, RUSCACEAE, SMILACACEAE, THEMIDACEAE, TOFIELDIACEAE) As here interpreted narrowly, the Liliaceae constitutes about 11 genera and 550 species, of the Northern Hemisphere. There has been much recent investigation and re-interpretation of evidence regarding the upper-level taxonomy of the Liliales, with strong suggestions that the broad Liliaceae recognized by Cronquist (1981) is artificial and polyphyletic. Cronquist (1993) himself concurs, at least to a degree: "we still await a comprehensive reorganization of the lilies into several families more comparable to other recognized families of angiosperms." Dahlgren & Clifford (1982) and Dahlgren, Clifford, & Yeo (1985) synthesized an early phase in the modern revolution of monocot taxonomy. Since then, additional research, especially molecular (Duvall et al. 1993, Chase et al. 1993, Bogler & Simpson 1995, and many others), has strongly validated the general lines (and many details) of Dahlgren's arrangement. The most recent synthesis (Kubitzki 1998a) is followed as the basis for familial and generic taxonomy of the lilies and their relatives (see summary below). References: Angiosperm Phylogeny Group (1998, 2003); Tamura in Kubitzki (1998a). Our “liliaceous” genera (members of orders placed in the Lilianae) are therefore divided as shown below, largely following Kubitzki (1998a) and some more recent molecular analyses. ALISMATALES TOFIELDIACEAE: Pleea, Tofieldia. LILIALES ALSTROEMERIACEAE: Alstroemeria COLCHICACEAE: Colchicum, Uvularia. LILIACEAE: Clintonia, Erythronium, Lilium, Medeola, Prosartes, Streptopus, Tricyrtis, Tulipa. MELANTHIACEAE: Amianthium, Anticlea, Chamaelirium, Helonias, Melanthium, Schoenocaulon, Stenanthium, Veratrum, Toxicoscordion, Trillium, Xerophyllum, Zigadenus. -

Chinquapin the Newsletter of the Southern Appalachian Botanical Society
chinquapin The Newsletter of the Southern Appalachian Botanical Society Volume 16, No. 4 Winter 2008 Happy Holidays from SABS Red spruce “hunkering down” for winter in the Great Smoky Mountains Photo by Scott Ranger 2 Chinquapin 16 (4) The Newsletter of the Southern Appalachian Botanical Society SABS Officers & Editors Conley K. McMullen, President Department of Biology, MSC 7801 Field Notes by Scott Ranger James Madison University Harrisonburg, VA 22807 3) Do weather conditions control flowering? (540) 568-3805 · fax (540) 568-3333 Three-birds Orchid Update I made a careful comparison of weather [email protected] conditions in 2007 (very hot with 14 days Howard S. Neufeld, Past President With another season of observing this > 90°F and 5 >100°F and dry with ~20% of Department of Biology ephemeral orchid at Pickett’s Mill Battlefield normal rainfall) and 2008 (nearly normal). 572 Rivers Street State Historic Site, I’ve come up with some The same flowering pattern occurred both Appalachian State University observations and questions. The photograph years. It seems weather, at least in these two Boone, NC 28608 below is illustrative for both. If anyone has years, didn’t have an effect on flowering. We (828) 262-2683 · fax (828) 262-2127 any answers, I’d love to hear them. counted a total of 460 stems in 2008, up [email protected] 61.5% from 2007. Weather probably had Charles N. Horn, Treasurer Observations: something to do with this. Biology Department • Even the smallest stems (>2 mm diameter 2100 College Street and >3 cm tall) have at least one well- 4) Is synchronicity overemphasized? I think Newberry College developed flower bud. -

Tofieldia Ulleungensis (Tofieldiaceae): a New Species, Endemic to Ulleungdo Island, Korea
Korean J. Pl. Taxon. 50(3): 343−350 (2020) pISSN 1225-8318 eISSN 2466-1546 https://doi.org/10.11110/kjpt.2020.50.3.343 Korean Journal of RESEARCH ARTICLE Plant Taxonomy Tofieldia ulleungensis (Tofieldiaceae): A new species, endemic to Ulleungdo Island, Korea Hyeryun JO, Balkrishna GHIMIRE, Young-Ho HA, Kang-Hyup LEE and Dong Chan SON* Division of Forest Biodiversity and Herbarium, Korea National Arboretum, Pocheon 11186, Korea (Received 19 August 2020; Revised 5 September 2020; Accepted 18 September 2020) ABSTRACT: Tofieldia ulleungensis, a new species of the genus Tofieldia from the Nari Basin on Ulleungdo Island, Korea, is described and illustrated. The new species is similar to T. yoshiiana var. koreana in terms of the plant height and in that it has having a long raceme, whitish tepals, and whitish stigma, but can be readily dis- tinguished from the latter by the presence of 1–2 linear cauline leaves, a slightly bent leaf apex, basal leaves which are twice as wide, a shorter pedicel, a revolute style, and crescent-shaped seeds. Keywords: Tofieldiaceae, Tofieldia ulleungensis, endemic, Ulleungdo Island, Korea The genus Tofieldia Huds. (Tofieldiaceae) comprised about recognized by Nakai (1911), but later he (Nakai, 1914) 12 species distributed in the subarctic, temperate, and transferred T. taquetii to T. fauriei. In 1916, Nakai reported a subtropical regions of the Northern Hemisphere (Chen and new species, T. nutans Willd. ex Schult.f., from Rhobong, Tamura, 2000; Yamazaki, 2002; Tamura et al., 2004, 2010, Pyeonganbuk-do, North Korea, and Chung (1957) recognized 2011). The species are morphologically characterized by 2- two species T. -

Narthecium Ossifragum – Moorlilie (Nartheciaceae), Blume Des Jahres
Jahrb. Bochumer Bot. Ver. 3 246-250 2012 Narthecium ossifragum – Moorlilie (Nartheciaceae ), Blume des Jahres 2011 ARMIN JAGEL & HUBERT SUMSER 1 Einleitung Im Jahr 2011 wurde die Moorlilie (andere Namen z. B. Beinbrech, Ährenlilie, Stablilie oder Knochenbruchgras) zur Blume des Jahres 2011 gewählt, zum letzten Mal von der bekannten Naturschützerin HANNELORE SCHMIDT , die auch die Rede zur Ernennung noch selbst verfasste (http://www.stiftung-naturschutz-hh.de/blume/rede2011.htm), aber kurz vor der Bekanntgabe mit 91 Jahren verstarb. Seit 1980 wird eine "Blume des Jahres" gekürt. Sie soll "Menschen immer wieder über den ökologischen Wert der Pflanzenwelt und über die Notwendigkeit des Schutzes aller bedrohten Arten informieren" (LOKI SCHMIDT , http://www.stiftung-naturschutz-hh.de/blumedj.htm). Mit der in Deutschland stark zurück- gegangenen Moorlilie will die LOKI -SCHMIDT -STIFTUNG den Lebensraum Moor ins öffentliche Bewusstsein rücken, stellvertretend für alle weiteren charakteristischen und ebenfalls oft gefährdeten Arten dieses stark gefährdeten Lebensraums. Abb. 1: Narthecium ossifragum , blühender Bestand im Further Moor zwischen Leichlingen und Langenfeld, Bergisches Land, NRW (05.07.2010, H. SUMSER ). Abb. 2: Narthecium ossifragum , Blütenstand (05.07.2010, H. SUMSER ). 2 Systematik Die Moorlilie gehörte traditionell in die artenreiche und sehr heterogene Pflanzenfamilie der Liliengewächse ( Liliaceae s. l.). Diese Großfamilie wurde nach und nach immer weiter aufgeteilt und man stellte Narthecium zunächst zu den Germergewächsen ( Melanthiaceae ), die z. B. in den deutschen Alpen noch mit dem Weißen Germer ( Veratrum album ) und der Simsenlilie ( Tofieldia calyculata ) vertreten sind. In jüngerer Zeit teilte man diese Familie erneut auf. Dabei wurde die Familie Nartheciaceae gebildet (MABBERLEY 2008). In Deutschland kommt davon neben der Moorlilie keine weitere Art vor. -

November 2020
November 19, 2020 crevice Alaska Rock Garden Society November Meeting: Alpines of the Pinnell Mountain National Recreation Trail By Marilyn Barker Saturday, November 21, 10 AM on Zoom [Zoom details to follow in separate email] Dear Members- I am looking forward to seeing all of you on our Zoom Meeting on Saturday. I think Marilyn’s program will be wonderful and informative. This will be the last meeting until next year! We will get information out to you about that meeting the first part of the month. Have a wonderful Holiday Season. Be safe and healthy, Florene Carney President Alaska Rock Garden Society (Zoom continued on page 2) Crevice is an occasional publication of the Alaska Rock Garden Society for small items. The masthead is an except from Eriophorum scheuchzeri by Rhonda Williams, used with permission. © Alaska Rock Garden Society 2020 PO Box 244136, Anchorage, AK 99524-4136 2 ARGS Crevice November 19, 2020 Alaska Rock Garden Society Membership Meeting November 21, 2020 ZOOM AGENDA Welcome Treasurer’s Report Approval of Minutes Seed Exchange Program – Marilyn Barker “Alpines of the Pinnell Mountain National Recreation Trail” Member Comments Adjourn Alaska Rock Garden Society Membership Meeting October 17, 2020 ZOOM 10:00 A.M. MINUTES Florene Carney called the meeting to order at 10:10 A.M. with a welcome to everyone joining our first ZOOM Membership Meeting. Jamie Smith, Secretary, gave a brief overview of the Oct 9, 2020 Exec Board minutes. Two important points: At that meeting, the current Executive Board UNANIMOUSLY agreed to remain in their current positions thru the 2020-2021 year to maintain continuity thru the COVID-19 Pandemic. -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Conserving Europe's Threatened Plants
Conserving Europe’s threatened plants Progress towards Target 8 of the Global Strategy for Plant Conservation Conserving Europe’s threatened plants Progress towards Target 8 of the Global Strategy for Plant Conservation By Suzanne Sharrock and Meirion Jones May 2009 Recommended citation: Sharrock, S. and Jones, M., 2009. Conserving Europe’s threatened plants: Progress towards Target 8 of the Global Strategy for Plant Conservation Botanic Gardens Conservation International, Richmond, UK ISBN 978-1-905164-30-1 Published by Botanic Gardens Conservation International Descanso House, 199 Kew Road, Richmond, Surrey, TW9 3BW, UK Design: John Morgan, [email protected] Acknowledgements The work of establishing a consolidated list of threatened Photo credits European plants was first initiated by Hugh Synge who developed the original database on which this report is based. All images are credited to BGCI with the exceptions of: We are most grateful to Hugh for providing this database to page 5, Nikos Krigas; page 8. Christophe Libert; page 10, BGCI and advising on further development of the list. The Pawel Kos; page 12 (upper), Nikos Krigas; page 14: James exacting task of inputting data from national Red Lists was Hitchmough; page 16 (lower), Jože Bavcon; page 17 (upper), carried out by Chris Cockel and without his dedicated work, the Nkos Krigas; page 20 (upper), Anca Sarbu; page 21, Nikos list would not have been completed. Thank you for your efforts Krigas; page 22 (upper) Simon Williams; page 22 (lower), RBG Chris. We are grateful to all the members of the European Kew; page 23 (upper), Jo Packet; page 23 (lower), Sandrine Botanic Gardens Consortium and other colleagues from Europe Godefroid; page 24 (upper) Jože Bavcon; page 24 (lower), Frank who provided essential advice, guidance and supplementary Scumacher; page 25 (upper) Michael Burkart; page 25, (lower) information on the species included in the database. -

Atlas of Rare Endemic Vascular Plants of the Arctic
Atlas of Rare Endemic Vascular Plants of the Arctic Technical Report No. 3 About CAFF Theprogram for the Conservation of Arctic Flora and Fauna (CAFF) of the Arctic Council was established lo address the special needs of Arctic ecosystems, species and thcir habitats in the rapid ly developing Arctic region. Itwas initiated as one of'four programs of the Arctic Environmental Protcction Strategy (AEPS) which was adopted by Canada, Denmark/Greenland, Finland, lceland, Norway, Russia, Swcdcn and the United States through a Ministeria! Declaration at Rovaniemi, Finland in 1991. Other programs initi ated under the AEPS and overlaken hy the Are.tie Council are the ArcticMonitoring and assessment Programme (AMAP), the program for Emergency Prevention, Preparcd ness and Response (EPPR) and the program for Protection of the Arctic Marine Envi ronment (PAME). Sinceits inaugural mccti.ng in Ottawa, Canada in 1992, the CAFF program has provided scientists, conscrvation managers and groups, and indigenous people of the north with a distinct forum in which lo tackle a wide range of Arctic conservation issues at the cir cumpolar level. CAFF's main goals, which are achieved in keeping with the concepts of sustainable developrnertt and utilisation, are: • to conserve Arctic Jlora and fauna, thcir diversity and thcir habitats; • to protect the Arctic ecosystems from threats; • to improve conservation management laws, reg ulations and practices for the Arclic; • to integrale Arctic interests into global conservation fora. CAFF operates rhrough a system of Designated Agencies and National Representatives responsible for CAFF in thcir rcspcctivc countries. CAFF also has an International Work ing Group wh.ith has met annually to assess progrcss and to develop Annual WorkPlans. -

FOH Newsletter 2012
FRIENDS Of The University Of Montana HERBARIUM Spring 2012 ....Bruce McCune MORE THAN VASCULAR AT MONTU By Andrea Pipp While perusing the vascular plant, moss, and lichen were the ecological relatives of mosses and incorporated specimens at MONTU you might see the name of Bruce them into his work. He relied on Mason Hale’s first edi- McCune. During his years at UM (1971-1979) Bruce con- tion of How to Know the Lichens and a long paper with tributed mounts of 62 vascular plant species and a larger keys titled “Lichens of the State of Washington” by Grace number of moss and lichen vouchers to the herbarium. Howard. He soon realized Hale’s book was very biased Bruce came to Missoula from the Midwest. Having toward eastern species, for even the most conspicuous grown up in Cincinnati and after completing his freshman lichens found on Mt. Sentinel weren’t in the book. Later year at Lawrence University in Appleton, Wisconsin, he discovered that Howard’s key contained as much mis- Bruce attended a wilderness botany class in northern Min- (Continued on page 7) nesota. There in the moss-carpeted forests he became quite interested in mosses and, in his words, was swept away by a remarkable young lady (Patricia Muir). Eager to escape the industrial winter of Appleton, Bruce fol- lowed Patricia to the University of Montana. That was 1971. The ‘new’ library was under construction, Hitch- cock and Cronquist, as a book, did not exist, and Botany stood on its own – a vibrant, active department, firmly ensconced in its own building. -

GENOME EVOLUTION in MONOCOTS a Dissertation
GENOME EVOLUTION IN MONOCOTS A Dissertation Presented to The Faculty of the Graduate School At the University of Missouri In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy By Kate L. Hertweck Dr. J. Chris Pires, Dissertation Advisor JULY 2011 The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled GENOME EVOLUTION IN MONOCOTS Presented by Kate L. Hertweck A candidate for the degree of Doctor of Philosophy And hereby certify that, in their opinion, it is worthy of acceptance. Dr. J. Chris Pires Dr. Lori Eggert Dr. Candace Galen Dr. Rose‐Marie Muzika ACKNOWLEDGEMENTS I am indebted to many people for their assistance during the course of my graduate education. I would not have derived such a keen understanding of the learning process without the tutelage of Dr. Sandi Abell. Members of the Pires lab provided prolific support in improving lab techniques, computational analysis, greenhouse maintenance, and writing support. Team Monocot, including Dr. Mike Kinney, Dr. Roxi Steele, and Erica Wheeler were particularly helpful, but other lab members working on Brassicaceae (Dr. Zhiyong Xiong, Dr. Maqsood Rehman, Pat Edger, Tatiana Arias, Dustin Mayfield) all provided vital support as well. I am also grateful for the support of a high school student, Cady Anderson, and an undergraduate, Tori Docktor, for their assistance in laboratory procedures. Many people, scientist and otherwise, helped with field collections: Dr. Travis Columbus, Hester Bell, Doug and Judy McGoon, Julie Ketner, Katy Klymus, and William Alexander. Many thanks to Barb Sonderman for taking care of my greenhouse collection of many odd plants brought back from the field.