Plastid Phylogenomic Analyses Resolve Tofieldiaceae As the Root of the Early Diverging Monocot Order Alismatales
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
1Lecture Notes 2013
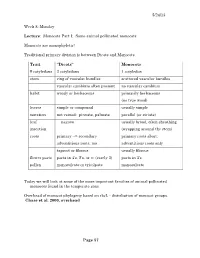
5/24/13 Week 8; Monday Lecture: Monocots Part I: Some animal pollinated monocots Monocots are monophyletic! Traditional primary division is between Dicots and Monocots Trait “Dicots” Monocots # cotyledons 2 cotyledons 1 cotyledon stem ring of vascular bundles scattered vascular bundles vascular cambium often present no vascular cambium habit woody or herbaceous primarily herbaceous (no true wood) leaves simple or compound usually simple venation net veined: pinnate, palmate parallel (or striate) leaf narrow usually broad, often sheathing insertion (wrapping around the stem) roots primary --> secondary primary roots abort; adventitious roots, too adventitious roots only taproot or fibrous usually fibrous flower parts parts in 4’s, 5’s, or ∞ (rarely 3) parts in 3’s pollen monosulcate or tricolpate monosulcate Today we will look at some of the more important families of animal pollinated monocots found in the temperate zone Overhead of monocot phylogeny based on rbcL - distribution of monocot groups. Chase et al. 2000, overhead Page 57 5/24/13 Lab only; limited discussion here. Show: “Plants are Cool, Too” video Araceae - Arum family (109 gen/2830 spp) 1) herbs (some epiphytes) 2) lvs simple or compound; broad and having an apparent petiole (‘pseudo-lamina’) development not same as in a dicot leaf blade 3) calcium oxalate crystals usually present – physical deterrent to herbivory 4) Inflorescence consisting of - spathe - bract (often colorful) surrounding the flowers - spadix - axis on which the flowers are borne (male above; female below, -

Halophila Ovalis, Ruppia Megacarpa and Posidonia Coriacea
Edith Cowan University Research Online Theses : Honours Theses 1998 The in vitro propagation of seagrasses : halophila ovalis, ruppia megacarpa and posidonia coriacea Melissa Grace Henry Edith Cowan University Follow this and additional works at: https://ro.ecu.edu.au/theses_hons Part of the Botany Commons, and the Marine Biology Commons Recommended Citation Henry, M. G. (1998). The in vitro propagation of seagrasses : halophila ovalis, ruppia megacarpa and posidonia coriacea. https://ro.ecu.edu.au/theses_hons/742 This Thesis is posted at Research Online. https://ro.ecu.edu.au/theses_hons/742 THE IN VITRO PROPAGATION OF SEAGRASSES: HALOPHILA OVALIS, RUPPIA MEGACARPA AND POSIDONIA CORIACEA MELISSA GRACE HENRY THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE AWARD OF B.SC. (BIOLOGICAL SCIENCE) HONOURS SCHOOL OF NATURAL SCIENCES EDITH COW AN UNIVERSITY JUNE 1998 ABSTRACT Seagrass communities are of high ecological and economic significance. They provide a nursery area for commercial and recreational juvenile fish and crustacea. Seagrasses also play an important role in influencing the structure and function of many estuarine and nearshore marine environments. Unfortunately, the decline of seagrasses, as a result of human impact, has increased in recent years. This decline has become a major problem throughout the world. Current methods used to restore degraded seagrass beds are limited, the most promising being transplanting material from healthy donor beds. This approach is expensive because it is labor intensive and damages the donor bed. Consequently, large scale transplanting programmes are not considered to be feasible. An alternative to using donor material may be found in the propagation of seagrasses. -

Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE
Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE LILIACEAE de Jussieu 1789 (Lily Family) (also see AGAVACEAE, ALLIACEAE, ALSTROEMERIACEAE, AMARYLLIDACEAE, ASPARAGACEAE, COLCHICACEAE, HEMEROCALLIDACEAE, HOSTACEAE, HYACINTHACEAE, HYPOXIDACEAE, MELANTHIACEAE, NARTHECIACEAE, RUSCACEAE, SMILACACEAE, THEMIDACEAE, TOFIELDIACEAE) As here interpreted narrowly, the Liliaceae constitutes about 11 genera and 550 species, of the Northern Hemisphere. There has been much recent investigation and re-interpretation of evidence regarding the upper-level taxonomy of the Liliales, with strong suggestions that the broad Liliaceae recognized by Cronquist (1981) is artificial and polyphyletic. Cronquist (1993) himself concurs, at least to a degree: "we still await a comprehensive reorganization of the lilies into several families more comparable to other recognized families of angiosperms." Dahlgren & Clifford (1982) and Dahlgren, Clifford, & Yeo (1985) synthesized an early phase in the modern revolution of monocot taxonomy. Since then, additional research, especially molecular (Duvall et al. 1993, Chase et al. 1993, Bogler & Simpson 1995, and many others), has strongly validated the general lines (and many details) of Dahlgren's arrangement. The most recent synthesis (Kubitzki 1998a) is followed as the basis for familial and generic taxonomy of the lilies and their relatives (see summary below). References: Angiosperm Phylogeny Group (1998, 2003); Tamura in Kubitzki (1998a). Our “liliaceous” genera (members of orders placed in the Lilianae) are therefore divided as shown below, largely following Kubitzki (1998a) and some more recent molecular analyses. ALISMATALES TOFIELDIACEAE: Pleea, Tofieldia. LILIALES ALSTROEMERIACEAE: Alstroemeria COLCHICACEAE: Colchicum, Uvularia. LILIACEAE: Clintonia, Erythronium, Lilium, Medeola, Prosartes, Streptopus, Tricyrtis, Tulipa. MELANTHIACEAE: Amianthium, Anticlea, Chamaelirium, Helonias, Melanthium, Schoenocaulon, Stenanthium, Veratrum, Toxicoscordion, Trillium, Xerophyllum, Zigadenus. -

Chinquapin the Newsletter of the Southern Appalachian Botanical Society
chinquapin The Newsletter of the Southern Appalachian Botanical Society Volume 16, No. 4 Winter 2008 Happy Holidays from SABS Red spruce “hunkering down” for winter in the Great Smoky Mountains Photo by Scott Ranger 2 Chinquapin 16 (4) The Newsletter of the Southern Appalachian Botanical Society SABS Officers & Editors Conley K. McMullen, President Department of Biology, MSC 7801 Field Notes by Scott Ranger James Madison University Harrisonburg, VA 22807 3) Do weather conditions control flowering? (540) 568-3805 · fax (540) 568-3333 Three-birds Orchid Update I made a careful comparison of weather [email protected] conditions in 2007 (very hot with 14 days Howard S. Neufeld, Past President With another season of observing this > 90°F and 5 >100°F and dry with ~20% of Department of Biology ephemeral orchid at Pickett’s Mill Battlefield normal rainfall) and 2008 (nearly normal). 572 Rivers Street State Historic Site, I’ve come up with some The same flowering pattern occurred both Appalachian State University observations and questions. The photograph years. It seems weather, at least in these two Boone, NC 28608 below is illustrative for both. If anyone has years, didn’t have an effect on flowering. We (828) 262-2683 · fax (828) 262-2127 any answers, I’d love to hear them. counted a total of 460 stems in 2008, up [email protected] 61.5% from 2007. Weather probably had Charles N. Horn, Treasurer Observations: something to do with this. Biology Department • Even the smallest stems (>2 mm diameter 2100 College Street and >3 cm tall) have at least one well- 4) Is synchronicity overemphasized? I think Newberry College developed flower bud. -

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

(BCF), Translocation Factor (TF) and Metal Enrichment Factor (MEF) Abilities of Aquatic Macrophyte Species Exposed to Metal Contaminated Wastewater
ISSN(Online): 2319-8753 ISSN (Print): 2347-6710 International Journal of Innovative Research in Science, Engineering and Technology (A High Impact Factor, Monthly, Peer Reviewed Journal) Visit: www.ijirset.com Vol. 8, Issue 1, January 2019 Evaluation of Bioaccumulation Factor (BAF), Bioconcentration Factor (BCF), Translocation Factor (TF) and Metal Enrichment Factor (MEF) Abilities of Aquatic Macrophyte Species Exposed to Metal Contaminated Wastewater S. S. Shingadgaon1, B.L. Chavan2 Research Scholar, Department of Environmental Science, School of Earth Sciences, Solapur University, Solapur, MS, India1 Former Professor and Head, Department of Environmental Science, Solapur University Solapur and presently working at Department of Environmental Science, Dr.Babasaheb Ambedkar Marathwada University, Aurangabad, MS, India 2 ABSTRACT: Wastewaters receiving aquatic bodies are quiet complex in terms of pollutants, the transport and interactions with heavy metals. This complexity is primarily due to high variability of pollutants, contaminants and related parameters. The macrophytes are plausible bio-indicators of the pollution load and level of metals within the aquatic systems than the wastewater or sediment analyses. The potential ability of aquatic macrophytes in natural water bodies receiving municipal sewage from Solapur city was assessed. Data from the studies on macrophytes exposed to a mixed test bath of metals and examined to know their potentialities to accumulate heavy metals for judging their suitability for phytoremediation technology -

Tofieldia Ulleungensis (Tofieldiaceae): a New Species, Endemic to Ulleungdo Island, Korea
Korean J. Pl. Taxon. 50(3): 343−350 (2020) pISSN 1225-8318 eISSN 2466-1546 https://doi.org/10.11110/kjpt.2020.50.3.343 Korean Journal of RESEARCH ARTICLE Plant Taxonomy Tofieldia ulleungensis (Tofieldiaceae): A new species, endemic to Ulleungdo Island, Korea Hyeryun JO, Balkrishna GHIMIRE, Young-Ho HA, Kang-Hyup LEE and Dong Chan SON* Division of Forest Biodiversity and Herbarium, Korea National Arboretum, Pocheon 11186, Korea (Received 19 August 2020; Revised 5 September 2020; Accepted 18 September 2020) ABSTRACT: Tofieldia ulleungensis, a new species of the genus Tofieldia from the Nari Basin on Ulleungdo Island, Korea, is described and illustrated. The new species is similar to T. yoshiiana var. koreana in terms of the plant height and in that it has having a long raceme, whitish tepals, and whitish stigma, but can be readily dis- tinguished from the latter by the presence of 1–2 linear cauline leaves, a slightly bent leaf apex, basal leaves which are twice as wide, a shorter pedicel, a revolute style, and crescent-shaped seeds. Keywords: Tofieldiaceae, Tofieldia ulleungensis, endemic, Ulleungdo Island, Korea The genus Tofieldia Huds. (Tofieldiaceae) comprised about recognized by Nakai (1911), but later he (Nakai, 1914) 12 species distributed in the subarctic, temperate, and transferred T. taquetii to T. fauriei. In 1916, Nakai reported a subtropical regions of the Northern Hemisphere (Chen and new species, T. nutans Willd. ex Schult.f., from Rhobong, Tamura, 2000; Yamazaki, 2002; Tamura et al., 2004, 2010, Pyeonganbuk-do, North Korea, and Chung (1957) recognized 2011). The species are morphologically characterized by 2- two species T. -

Global Seagrass Distribution and Diversity: a Bioregional Model ⁎ F
Journal of Experimental Marine Biology and Ecology 350 (2007) 3–20 www.elsevier.com/locate/jembe Global seagrass distribution and diversity: A bioregional model ⁎ F. Short a, , T. Carruthers b, W. Dennison b, M. Waycott c a Department of Natural Resources, University of New Hampshire, Jackson Estuarine Laboratory, Durham, NH 03824, USA b Integration and Application Network, University of Maryland Center for Environmental Science, Cambridge, MD 21613, USA c School of Marine and Tropical Biology, James Cook University, Townsville, 4811 Queensland, Australia Received 1 February 2007; received in revised form 31 May 2007; accepted 4 June 2007 Abstract Seagrasses, marine flowering plants, are widely distributed along temperate and tropical coastlines of the world. Seagrasses have key ecological roles in coastal ecosystems and can form extensive meadows supporting high biodiversity. The global species diversity of seagrasses is low (b60 species), but species can have ranges that extend for thousands of kilometers of coastline. Seagrass bioregions are defined here, based on species assemblages, species distributional ranges, and tropical and temperate influences. Six global bioregions are presented: four temperate and two tropical. The temperate bioregions include the Temperate North Atlantic, the Temperate North Pacific, the Mediterranean, and the Temperate Southern Oceans. The Temperate North Atlantic has low seagrass diversity, the major species being Zostera marina, typically occurring in estuaries and lagoons. The Temperate North Pacific has high seagrass diversity with Zostera spp. in estuaries and lagoons as well as Phyllospadix spp. in the surf zone. The Mediterranean region has clear water with vast meadows of moderate diversity of both temperate and tropical seagrasses, dominated by deep-growing Posidonia oceanica. -

Narthecium Ossifragum – Moorlilie (Nartheciaceae), Blume Des Jahres
Jahrb. Bochumer Bot. Ver. 3 246-250 2012 Narthecium ossifragum – Moorlilie (Nartheciaceae ), Blume des Jahres 2011 ARMIN JAGEL & HUBERT SUMSER 1 Einleitung Im Jahr 2011 wurde die Moorlilie (andere Namen z. B. Beinbrech, Ährenlilie, Stablilie oder Knochenbruchgras) zur Blume des Jahres 2011 gewählt, zum letzten Mal von der bekannten Naturschützerin HANNELORE SCHMIDT , die auch die Rede zur Ernennung noch selbst verfasste (http://www.stiftung-naturschutz-hh.de/blume/rede2011.htm), aber kurz vor der Bekanntgabe mit 91 Jahren verstarb. Seit 1980 wird eine "Blume des Jahres" gekürt. Sie soll "Menschen immer wieder über den ökologischen Wert der Pflanzenwelt und über die Notwendigkeit des Schutzes aller bedrohten Arten informieren" (LOKI SCHMIDT , http://www.stiftung-naturschutz-hh.de/blumedj.htm). Mit der in Deutschland stark zurück- gegangenen Moorlilie will die LOKI -SCHMIDT -STIFTUNG den Lebensraum Moor ins öffentliche Bewusstsein rücken, stellvertretend für alle weiteren charakteristischen und ebenfalls oft gefährdeten Arten dieses stark gefährdeten Lebensraums. Abb. 1: Narthecium ossifragum , blühender Bestand im Further Moor zwischen Leichlingen und Langenfeld, Bergisches Land, NRW (05.07.2010, H. SUMSER ). Abb. 2: Narthecium ossifragum , Blütenstand (05.07.2010, H. SUMSER ). 2 Systematik Die Moorlilie gehörte traditionell in die artenreiche und sehr heterogene Pflanzenfamilie der Liliengewächse ( Liliaceae s. l.). Diese Großfamilie wurde nach und nach immer weiter aufgeteilt und man stellte Narthecium zunächst zu den Germergewächsen ( Melanthiaceae ), die z. B. in den deutschen Alpen noch mit dem Weißen Germer ( Veratrum album ) und der Simsenlilie ( Tofieldia calyculata ) vertreten sind. In jüngerer Zeit teilte man diese Familie erneut auf. Dabei wurde die Familie Nartheciaceae gebildet (MABBERLEY 2008). In Deutschland kommt davon neben der Moorlilie keine weitere Art vor. -

Reassessment of Seagrass Species in the Marshall Islands1
Micronesica 2016-04: 1–10 Reassessment of Seagrass Species in the Marshall Islands 1 ROY T. TSUDA Department of Natural Sciences, Bishop Museum, 1525 Bernice Street, Honolulu, HI 96817, USA [email protected] NADIERA SUKHRAJ U.S. Fish and Wildlife Service, Pacific Islands Fish and Wildlife Office, 300 Ala Moana Blvd., Honolulu, HI 96850, USA [email protected] Abstract—Recent collections of specimens of Halophila gaudichaudii J. Kuo, previously identified as Halophila minor (Zollinger) den Hartog, from Kwajalein Atoll in September 2016 and the archiving of the specimens at BISH validate the previous observation of this seagrass genus in the Marshall Islands. Previously, no voucher specimen was available for examination. Molecular analyses of the Kwajalein Halophila specimens may demonstrate conspecificity with Halophila nipponica J. Kuo with H. gaudichaudii relegated as a synonym. Herbarium specimens of Cymodocea rotundata Ehrenberg and Hemprich ex Ascherson from Majuro Atoll were found at BISH and may represent the only specimens from the Marshall Islands archived in a herbarium. Cymodocea rotundata, however, has been documented in past literature and archived via digital photos in its natural habitat in Majuro. The previous validation of Thalassia hemprichii (Ehrenberg) Ascherson with specimens, and the recent validation of Halophila gaudichaudii and Cymodocea rotundata with specimens reaffirm the low coral atolls and islands of the Marshall Islands as the eastern limit for the three species in the Pacific Ocean. Introduction In a review of the seagrasses in Micronesia, Tsuda et al. (1977) reported nine species of seagrasses in Micronesia with new records of Thalassodendron ciliatum (Forsskål) den Hartog from Palau, and Syringodium isoetifolium (Ascherson) Dandy and Cymodocea serrulata (R. -

Introduction to Common Native & Invasive Freshwater Plants in Alaska
Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska Cover photographs by (top to bottom, left to right): Tara Chestnut/Hannah E. Anderson, Jamie Fenneman, Vanessa Morgan, Dana Visalli, Jamie Fenneman, Lynda K. Moore and Denny Lassuy. Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska This document is based on An Aquatic Plant Identification Manual for Washington’s Freshwater Plants, which was modified with permission from the Washington State Department of Ecology, by the Center for Lakes and Reservoirs at Portland State University for Alaska Department of Fish and Game US Fish & Wildlife Service - Coastal Program US Fish & Wildlife Service - Aquatic Invasive Species Program December 2009 TABLE OF CONTENTS TABLE OF CONTENTS Acknowledgments ............................................................................ x Introduction Overview ............................................................................. xvi How to Use This Manual .................................................... xvi Categories of Special Interest Imperiled, Rare and Uncommon Aquatic Species ..................... xx Indigenous Peoples Use of Aquatic Plants .............................. xxi Invasive Aquatic Plants Impacts ................................................................................. xxi Vectors ................................................................................. xxii Prevention Tips .................................................... xxii Early Detection and Reporting -

Aquatic Vascular Plant Species Distribution Maps
Appendix 11.5.1: Aquatic Vascular Plant Species Distribution Maps These distribution maps are for 116 aquatic vascular macrophyte species (Table 1). Aquatic designation follows habitat descriptions in Haines and Vining (1998), and includes submergent, floating and some emergent species. See Appendix 11.4 for list of species. Also included in Appendix 11.4 is the number of HUC-10 watersheds from which each taxon has been recorded, and the county-level distributions. Data are from nine sources, as compiled in the MABP database (plus a few additional records derived from ancilliary information contained in reports from two fisheries surveys in the Upper St. John basin organized by The Nature Conservancy). With the exception of the University of Maine herbarium records, most locations represent point samples (coordinates were provided in data sources or derived by MABP from site descriptions in data sources). The herbarium data are identified only to township. In the species distribution maps, town-level records are indicated by center-points (centroids). Figure 1 on this page shows as polygons the towns where taxon records are identified only at the town level. Data Sources: MABP ID MABP DataSet Name Provider 7 Rare taxa from MNAP lake plant surveys D. Cameron, MNAP 8 Lake plant surveys D. Cameron, MNAP 35 Acadia National Park plant survey C. Greene et al. 63 Lake plant surveys A. Dieffenbacher-Krall 71 Natural Heritage Database (rare plants) MNAP 91 University of Maine herbarium database C. Campbell 183 Natural Heritage Database (delisted species) MNAP 194 Rapid bioassessment surveys D. Cameron, MNAP 207 Invasive aquatic plant records MDEP Maps are in alphabetical order by species name.