Guardant Health Q4 2020 Earnings Call Operator: Ladies And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guardant Health, Inc. Form 8-K Current Event Report Filed 2019-01
SECURITIES AND EXCHANGE COMMISSION FORM 8-K Current report filing Filing Date: 2019-01-07 | Period of Report: 2019-01-07 SEC Accession No. 0001628280-19-000132 (HTML Version on secdatabase.com) FILER Guardant Health, Inc. Mailing Address Business Address 505 PENOBSCOT DR. 505 PENOBSCOT DR. CIK:1576280| IRS No.: 454139254 | State of Incorp.:DE | Fiscal Year End: 1231 REDWOOD CITY CA 94063 REDWOOD CITY CA 94063 Type: 8-K | Act: 34 | File No.: 001-38683 | Film No.: 19512177 855-698-8887 SIC: 8071 Medical laboratories Copyright © 2019 www.secdatabase.com. All Rights Reserved. Please Consider the Environment Before Printing This Document UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of report (Date of earliest event reported): January 7, 2019 GUARDANT HEALTH, INC. (Exact name of registrant as specified in its charter) Delaware 0001-576280 45-4139254 (State or other jurisdiction (Commission (I.R.S. Employer of incorporation or organization) File Number) Identification No.) 505 Penobscot Dr. Redwood City, California 94063 (Address of principal executive offices) (Zip Code) 855-698-8887 (Registrant’s telephone number, include area code) N/A (Former Name or Former Address, if Changed Since Last Report) Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions: ¨ Written communications pursuant to Rule 425 under -

797 Circulating Tumor DNA and Circulating Tumor Cells for Cancer
Medical Policy Circulating Tumor DNA and Circulating Tumor Cells for Cancer Management (Liquid Biopsy) Table of Contents • Policy: Commercial • Coding Information • Information Pertaining to All Policies • Policy: Medicare • Description • References • Authorization Information • Policy History • Endnotes Policy Number: 797 BCBSA Reference Number: 2.04.141 Related Policies Biomarkers for the Diagnosis and Cancer Risk Assessment of Prostate Cancer, #336 Policy1 Commercial Members: Managed Care (HMO and POS), PPO, and Indemnity Plasma-based comprehensive somatic genomic profiling testing (CGP) using Guardant360® for patients with Stage IIIB/IV non-small cell lung cancer (NSCLC) is considered MEDICALLY NECESSARY when the following criteria have been met: Diagnosis: • When tissue-based CGP is infeasible (i.e., quantity not sufficient for tissue-based CGP or invasive biopsy is medically contraindicated), AND • When prior results for ALL of the following tests are not available: o EGFR single nucleotide variants (SNVs) and insertions and deletions (indels) o ALK and ROS1 rearrangements o PDL1 expression. Progression: • Patients progressing on or after chemotherapy or immunotherapy who have never been tested for EGFR SNVs and indels, and ALK and ROS1 rearrangements, and for whom tissue-based CGP is infeasible (i.e., quantity not sufficient for tissue-based CGP), OR • For patients progressing on EGFR tyrosine kinase inhibitors (TKIs). If no genetic alteration is detected by Guardant360®, or if circulating tumor DNA (ctDNA) is insufficient/not detected, tissue-based genotyping should be considered. Other plasma-based CGP tests are considered INVESTIGATIONAL. CGP and the use of circulating tumor DNA is considered INVESTIGATIONAL for all other indications. 1 The use of circulating tumor cells is considered INVESTIGATIONAL for all indications. -

Guardant Health, Inc. Form 10-Q Quarterly Report Filed 2021-08-05
SECURITIES AND EXCHANGE COMMISSION FORM 10-Q Quarterly report pursuant to sections 13 or 15(d) Filing Date: 2021-08-05 | Period of Report: 2021-06-30 SEC Accession No. 0001576280-21-000080 (HTML Version on secdatabase.com) FILER Guardant Health, Inc. Mailing Address Business Address 505 PENOBSCOT DR. 505 PENOBSCOT DR. CIK:1576280| IRS No.: 454139254 | State of Incorp.:DE | Fiscal Year End: 1231 REDWOOD CITY CA 94063 REDWOOD CITY CA 94063 Type: 10-Q | Act: 34 | File No.: 001-38683 | Film No.: 211148786 855-698-8887 SIC: 8071 Medical laboratories Copyright © 2021 www.secdatabase.com. All Rights Reserved. Please Consider the Environment Before Printing This Document UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, DC 20549 _____________________ FORM 10-Q _____________________ (Mark One) ☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the quarterly period ended June 30, 2021 OR ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission File Number: 001-38683 _____________________ GUARDANT HEALTH, INC. (Exact Name of Registrant as Specified in its Charter) _____________________ Delaware 45-4139254 (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification No.) 505 Penobscot Dr. Redwood City, California, 94063 Registrant’s telephone number, including area code: (855) 698-8887 _______________ Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -
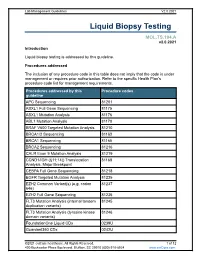
Liquid Biopsy Testing
Lab Management Guidelines V2.0.2021 Liquid Biopsy Testing MOL.TS.194.A v2.0.2021 Introduction Liquid biopsy testing is addressed by this guideline. Procedures addressed The inclusion of any procedure code in this table does not imply that the code is under management or requires prior authorization. Refer to the specific Health Plan's procedure code list for management requirements. Procedures addressed by this Procedure codes guideline APC Sequencing 81201 ASXL1 Full Gene Sequencing 81175 ASXL1 Mutation Analysis 81176 ABL1 Mutation Analysis 81170 BRAF V600 Targeted Mutation Analysis 81210 BRCA1/2 Sequencing 81163 BRCA1 Sequencing 81165 BRCA2 Sequencing 81216 CALR Exon 9 Mutation Analysis 81219 CCND1/IGH (t(11;14)) Translocation 81168 Analysis, Major Breakpoint CEBPA Full Gene Sequencing 81218 EGFR Targeted Mutation Analysis 81235 EZH2 Common Variant(s) (e.g. codon 81237 646) EZH2 Full Gene Sequencing 81236 FLT3 Mutation Analysis (internal tandem 81245 duplication variants) FLT3 Mutation Analysis (tyrosine kinase 81246 domain variants) FoundationOne Liquid CDx 0239U Guardant360 CDx 0242U ©2021 eviCore healthcare. All Rights Reserved. 1 of 12 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Lab Management Guidelines V2.0.2021 Procedures addressed by this Procedure codes guideline Hematolymphoid Neoplasm Molecular 81450 Profiling; 5-50 genes IDH1 Mutation Analysis 81120 IDH2 Mutation Analysis 81121 IGH@/BCL2 (t(14;18)) Translocation 81278 Analysis, Major Breakpoint Region (MBR) and Minor Cluster Region (mcr) Breakpoints JAK2 Targeted Mutation Analysis (e.g 81279 exons 12 and 13) JAK2 V617F Targeted Mutation Analysis 81270 KIT Targeted Sequence Analysis 81272 KIT D816 Targeted Mutation Analysis 81273 KRAS Exon 2 Targeted Mutation Analysis 81275 KRAS Targeted Mutation Analysis, 81276 Additional Variants MGMT Promoter Methylation Analysis 81287 MLH1 Sequencing 81292 Molecular Tumor Marker Test 81400 81401 81402 g 81403 n i 81405 t 81406 s e T 81407 81408 y s 81479 p o Molecular Tumor Marker Test 88271 i B MPL Common Variants (e.g. -

Promises and Pitfalls of Using Liquid Biopsy for Precision Medicine Giovanna Rossi1 and Michail Ignatiadis2
Published OnlineFirst May 20, 2019; DOI: 10.1158/0008-5472.CAN-18-3402 Cancer Review Research Promises and Pitfalls of Using Liquid Biopsy for Precision Medicine Giovanna Rossi1 and Michail Ignatiadis2 Abstract New sensitive assays are currently available for the detec- Multiple studies are underway to assess the clinical utility of tion of circulating tumor DNA (ctDNA) and circulating tumor CTC and ctDNA in different settings (treatment-na€ve vs. cells (CTC). However, there remains a need for standardiza- resistant, adjuvant vs. metastatic) and for different treatment tion of preanalytical issues and cross-platform comparison modalities (systemic therapy, surgery, radiation therapy). studies. Liquid biopsies are being evaluated for treatment This review aims to map the challenges that remain to be selection, for monitoring disease response and resistance, for addressed before liquid biopsies can be widely used for cancer tracking minimal residual disease, and for cancer diagnosis. management. Introduction applications of CTCs and ctDNA that are currently being explored are summarized in Fig. 1A. In the era of precision medicine, liquid biopsies are increasingly being studied as a tool that can capture tumor evolution in real time and thus guide systemic treatment. In this article, we will refer to the analysis of circulating tumor DNA (ctDNA) and circulating Challenges Associated with Preanalytical tumor cells (CTC) only and we will not cover other liquid biopsy Issues and the Analytical Validity of Liquid biomarkers such as circulating RNAs, proteins, metabolites, and Biopsy Assays exosomes. For CTC and ctDNA assays, there is a need to standardize Sampling a patient's blood may give information about the preanalytical variables and for cross-platform comparison stud- genomic profile of a given cancer (1–3) and provide an assess- ies. -

Liquid Biopsy Analysis in Clinical Practice: Focus on Lung Cancer
Review Liquid Biopsy Analysis in Clinical Practice: Focus on Lung Cancer Pasquale Pisapia 1 , Francesco Pepe 1, Antonino Iaccarino 1, Roberta Sgariglia 1, Mariantonia Nacchio 1, Gianluca Russo 1 , Gianluca Gragnano 1, Elalah Mosaieby 2, Giancarlo Troncone 1,* and Umberto Malapelle 1 1 Department of Public Health, University of Naples Federico II, 80131 Naples, Italy; [email protected] (P.P.); [email protected] (F.P.); [email protected] (A.I.); [email protected] (R.S.); [email protected] (M.N.); [email protected] (G.R.); [email protected] (G.G.); [email protected] (U.M.) 2 Department of Cellular and Molecular Biology, University of Mazandaran, Mazandaran 48175-866, Iran; [email protected] * Correspondence: [email protected] Abstract: Lung cancer is the leading cause of cancer death worldwide. Despite the emergence of highly effective targeted therapies, up to 30% of advanced stage non-small cell lung cancer (NSCLC) patients do not undergo tissue molecular testing because of scarce tissue availability. Liquid biopsy, on the other hand, offers these patients a valuable opportunity to receive the best treatment options in a timely manner. Indeed, besides being much faster and less invasive than conventional tissue- based analysis, it can also yield specific information about the genetic make-up and evolution of patients’ tumors. However, several issues, including lack of standardized protocols for sample collection, processing, and interpretation, still need to be addressed before liquid biopsy can be Citation: Pisapia, P.; Pepe, F.; fully incorporated into routine oncology practice. Here, we reviewed the most important challenges Iaccarino, A.; Sgariglia, R.; Nacchio, hindering the implementation of liquid biopsy in oncology practice, as well as the great advantages M.; Russo, G.; Gragnano, G.; Mosaieby, E.; Troncone, G.; Malapelle, of this approach for the treatment of NSCLC patients. -

Accelerating the Future of Liquid Biopsy
Accelerating the future of liquid biopsy Ultra-low mutation detection solutions from sample prep to data analysis The exciting potential of liquid biopsy in oncology research Currently, the most common strategy for characterizing the Recent studies have shown the utility of liquid biopsies for: genetic makeup of a tumor is the extraction, or biopsy, of a sample of the affected tissue. Tissue biopsies, however, • Enhancing understanding of tumorigenesis, metastasis, can be painful, risky, and in some cases not feasible when and therapy resistance a tumor is difficult to access. Furthermore, tissue biopsies • Detection of cancer at early stages when treatment may are not a viable monitoring technique as they cannot be be most successful repeated, and they may not be representative of the entire tumor due to tumor heterogeneity. • Monitoring of cancer development, disease progression, and recurrence Liquid biopsy is an emerging area of clinical research, • Tracking response or resistance during and after particularly in the context of cancer. As a minimally invasive treatment to allow for adjustments in real time complementary or alternative approach to tissue biopsies, liquid biopsies are less risky, painful, and costly, and are increasingly being used to analyze biomarkers in liquid samples, such as blood. 2 Unlock the potential in your liquid biopsy samples MagMAX cell-free nucleic acid isolation kits Liquid biopsies most often utilize cell-free DNA (cfDNA) that is derived from both normal and cancerous cells. The tumor-only supply of DNA in the bloodstream is more commonly referred to as circulating tumor DNA (ctDNA), which is loaded with information about a tumor that would otherwise be difficult to access. -

Medtechs Rake in the Venture Cash
January 14, 2021 Medtechs rake in the venture cash Elizabeth Cairns In terms of private investments, 2020 was the year of the liquid biopsy. Looking at the cash raised by private medical device companies last year you would be hard pressed to find any evidence of a pandemic at all. Venture investors poured $6.4bn into the sector, with the particularly strong performance in the second half putting the total markedly above 2019’s. And some of the very biggest investments have already paid off handsomely. The liquid biopsy developers Grail and Thrive hooked nine-figure rounds and were promptly bought for billions of dollars each, by Illumina and Exact Sciences respectively. The VCs backing their fellow cancer blood test specialists Freenome and Caris Life Sciences doubtless also had takeovers in mind. In fact diagnostics companies dominate the top 10 rounds. In addition to the four liquid biopsy groups, Everlywell, Karius and Oxford Nanopore Technologies – two of which are working on tests for Covid-19 – also enjoyed sizeable cash injections. Diagnostics developers, with their relatively cheap and easily scalable technology, are always appealing to VCs, but 2020 was something special. Perhaps the wider environment pushed investors towards these safe bets. But there is a growing consensus that liquid biopsies, though new, are a technology on which oncologists will come to rely. The first US approvals for such tests, from Guardant Health and Roche, were awarded in August, and a third, from Grail, is set for launch without FDA oversight in the second quarter of 2021. Investors’ interest in liquid biopsy plays might also have been influenced by Covid-19. -

Monitoring Trace Levels of Ctdna Using Integration of Variant Reads
Monitoring trace levels of ctDNA using 1 integration of variant reads 2 3 Jonathan C. M. Wan 4 Supervisor: Dr Nitzan Rosenfeld 5 6 Cancer Research UK Cambridge Institute 7 8 University of Cambridge This dissertation is submitted for the degree of 9 Doctor of Philosophy 10 Trinity College May 2019 11 Abstract 12 In patients with early-stage cancer, ctDNA detection rates can be low due to the presence of 13 few or no copies of any individual mutation in each sample. Sensitivity may be increased 14 by collecting larger plasma volumes, but this is not feasible in practice. Although cancers 15 typically have thousands of mutations in their genome, previous analyses measured only 16 individual or up to 32 tumour-specific mutations in plasma. 17 Here, we demonstrate that sensitivity can be greatly enhanced for any given input DNA 18 mass by analysing a large number of mutations via sequencing. We sequenced in plasma 2 4 19 10 -10 mutated loci per patient, using custom capture panels, whole exome (WES) or 20 whole genome sequencing (WGS). We developed a method for INtegration of VAriant Reads 21 (INVAR) that aggregates reads carrying tumour mutations across multiple mutant loci, and 22 assigns confidence to error-suppressed reads based on mutation context, fragment length and 23 tumour representation. This workflow combines a number of concepts in a novel approach in 24 order to quantify ctDNA with maximal sensitivity. 25 We applied INVARto plasma sequencing data from 45 patients with stage II-IV melanoma 26 and 26 healthy individuals. ctDNA was detected to 1 mutant molecule per million, and tu- 3 27 mour volumes of ~1cm . -

Liquid Biopsy: a Family of Possible Diagnostic Tools
diagnostics Review Liquid Biopsy: A Family of Possible Diagnostic Tools Battistelli Michela Department of Biomolecular Sciences, Carlo Bo Urbino University, 61029 Urbino, Italy; [email protected] Abstract: Liquid biopsies could be considered an excellent diagnostic tool, in different physiological or pathological conditions. The possibility of using liquid biopsies for non-invasive clinical purposes is quite an old idea: indeed many years ago it was already being used in the field of non-invasive prenatal tests (NIPT) for autosomal fetal aneuploidy evaluation. In 1997 Lo et al. had identified fetal DNA in maternal plasma and serum, showing that about 10–15% of cfDNA in maternal plasma is derived from the placenta, and biologic fluid represents an important and non-invasive technique to evaluate state diseases and possible therapies. Nowadays, several body fluids, such as blood, urine, saliva and other patient samples, could be used as liquid biopsy for clinical non-invasive evaluation. These fluids contain numerous and various biomarkers and could be used for the evaluation of pathological and non-pathological conditions. In this review we will analyze the different types of liquid biopsy, their potential role in clinical diagnosis and the functional involvement of extracellular vesicles in these fluids as carriers. Keywords: liquid biopsies; extracellular vesicles; tumor dynamic evaluation 1. Introduction Several biological body fluids, such as blood, urine, saliva, breast milk and other Citation: Michela, B. Liquid Biopsy: patient samples, can be used for clinical investigations, because they contain numerous A Family of Possible Diagnostic Tools. biomarkers [1]. The biomarkers found in these liquids can be DNA, RNA, proteins or even Diagnostics 2021, 11, 1391. -

Liquid Biopsies in Solid Cancers: Implementation in a Nordic Healthcare System
cancers Review Liquid Biopsies in Solid Cancers: Implementation in a Nordic Healthcare System Oddmund Nordgård 1,2,*,†, Rakel Brendsdal Forthun 3,4,†, Morten Lapin 1 , Bjørn Henning Grønberg 5,6, Karl Henning Kalland 7,8 , Reidun Kristin Kopperud 7, Liv Cecilie Vestrheim Thomsen 7 , Kjersti Tjensvoll 1, Bjørnar Gilje 1, Bjørn Tore Gjertsen 7,9 and Randi Hovland 3,4 1 Department of Hematology and Oncology, Stavanger University Hospital, 4011 Stavanger, Norway; [email protected] (M.L.); [email protected] (K.T.); [email protected] (B.G.) 2 Department of Chemistry, Bioscience and Environmental Engineering, University of Stavanger, 4021 Stavanger, Norway 3 Department of Medical Genetics, Haukeland University Hospital, 5021 Bergen, Norway; [email protected] (R.B.F.); [email protected] (R.H.) 4 Section of Cancer Genomics, Haukeland University Hospital, 5021 Bergen, Norway 5 Department of Clinical and Molecular Medicine, NTNU, Norwegian University of Science and Technology, 7491 Trondheim, Norway; [email protected] 6 Department of Oncology, St. Olav’s Hospital, Trondheim University Hospital, 7030 Trondheim, Norway 7 Centre for Cancer Biomarkers CCBIO, Department of Clinical Science, University of Bergen, 5021 Bergen, Norway; [email protected] (K.H.K.); [email protected] (R.K.K.); [email protected] (L.C.V.T.); [email protected] (B.T.G.) 8 Department of Microbiology, Haukeland University Hospital, 5021 Bergen, Norway 9 Department of Internal Medicine, Hematology Section, Haukeland University Hospital, 5021 Bergen, Norway Citation: Nordgård, O.; Brendsdal * Correspondence: [email protected] Forthun, R.; Lapin, M.; Grønberg, † Shared first-authorship. -

Liquid Biopsy Is at the Core of Our Mission to Conquer Cancer with Data
guardanthealth.com • [email protected] • +1.812.731.2203 CORPORATE BACKGROUNDER LIQUID BIOPSY IS AT THE CORE OF OUR MISSION TO CONQUER CANCER WITH DATA Guardant Health is a leading precision oncology company focused on helping conquer cancer globally through use of our proprietary blood tests, vast data sets, and advanced analytics. A company uniquely positioned to help patients across the entire cancer care continuum At Guardant Health, we believe liquid biopsy is at the center of transforming cancer care by unlocking data that will help patients at all stages of the disease. While we’ve made great strides to help advanced cancer patients, our vision since inception has been to detect cancer early, when patient survival rates can be impacted most. We are dedicated to helping patients across the cancer care continuum live longer, healthier lives. 700K+ 15M+ 65M+ Advanced Cancer Early-Stage Patients Asymptomatic and Patients and Survivors High Risk Screening Guardant360® CDx liquid biopsy LUNAR-1 program (in development) LUNAR-2 program (in development) (Estimated number of patients in the US) Guardant360® CDx liquid biopsy is poised to bring the promise of precision oncology to more patients Guardant360® CDx is the first FDA-approved liquid biopsy for comprehensive genomic profiling (CGP) in advanced cancer patients across all solid cancers, and for use as a companion diagnostic for patients who may benefit from Tagrisso® (osimertinib). We believe the ease of our blood test together with approval will help widen adoption of CGP and enable more patients to receive potentially life-changing precision medicines. In 2014, we introduced the Guardant360 laboratory developed test (LDT), the first-in-kind liquid biopsy to comprehensively sequence a patient's cancer to reveal actionable mutations.