Liquid Biopsy Analysis in Clinical Practice: Focus on Lung Cancer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Next Big Thing in Chromatography?
The Next Big Thing In Chromatography? Find out how microscale chromatography is making a big splash in analytical science Please click the circles to navigate Technology Perfecting Chromatography Technical & with a Real Proteomic Peaks in Silicon Application Edge Separations Valley Notes PERFECTING CHROMATOGRAPHY TECHNOLOGY WITH TECHNICAL & PROTEOMIC PEAKS IN SILICON A REAL EDGE APPLICATION NOTES SEPARATIONS VALLEY μPAC™ at the Edge As uptake of μPAC™ grows, the technology is contributing to exciting advances in biology and beyond. Here are just three projects that hit the headlines in 2019… Tree of Life At the EMBL Wellcome Genome Campus Conference in March 2019, the Matthias Mann Group (Max Planck Institute, Munich, Germany) presented the quantitative proteome atlas of 100 organisms across all three kingdoms, fingerprinted thanks to the high retention time stability and reproducibility of the μPAC™. The Tree of Life is the largest open access proteome data set ever reported, with more than 250,000 proteins, and growing. Labs around the world can use the open access database together with μPAC™ and machine learning to predict a retention time fingerprint for each individual protein in the Tree of Life – the potential for hyper-resolved target data deconvolution is immense. Doubling Up on Single Cells Single-cell proteomics is poised to revolutionize many fields of biological research, with important implications for therapeutics, discovery, genomics and translational research. In a presentation titled “Double protein IDs in Single Cell protocols”, Karl Mechtler (Institute of Molecular Pathology, Vienna) explained how his group have identified 3,500 Brussel in late 2010 and set up shop as a microfluidics consulting proteins in a 10 ng HeLa cell sample using the μPAC™ Technology with a Real Edge boutique. -

797 Circulating Tumor DNA and Circulating Tumor Cells for Cancer
Medical Policy Circulating Tumor DNA and Circulating Tumor Cells for Cancer Management (Liquid Biopsy) Table of Contents • Policy: Commercial • Coding Information • Information Pertaining to All Policies • Policy: Medicare • Description • References • Authorization Information • Policy History • Endnotes Policy Number: 797 BCBSA Reference Number: 2.04.141 Related Policies Biomarkers for the Diagnosis and Cancer Risk Assessment of Prostate Cancer, #336 Policy1 Commercial Members: Managed Care (HMO and POS), PPO, and Indemnity Plasma-based comprehensive somatic genomic profiling testing (CGP) using Guardant360® for patients with Stage IIIB/IV non-small cell lung cancer (NSCLC) is considered MEDICALLY NECESSARY when the following criteria have been met: Diagnosis: • When tissue-based CGP is infeasible (i.e., quantity not sufficient for tissue-based CGP or invasive biopsy is medically contraindicated), AND • When prior results for ALL of the following tests are not available: o EGFR single nucleotide variants (SNVs) and insertions and deletions (indels) o ALK and ROS1 rearrangements o PDL1 expression. Progression: • Patients progressing on or after chemotherapy or immunotherapy who have never been tested for EGFR SNVs and indels, and ALK and ROS1 rearrangements, and for whom tissue-based CGP is infeasible (i.e., quantity not sufficient for tissue-based CGP), OR • For patients progressing on EGFR tyrosine kinase inhibitors (TKIs). If no genetic alteration is detected by Guardant360®, or if circulating tumor DNA (ctDNA) is insufficient/not detected, tissue-based genotyping should be considered. Other plasma-based CGP tests are considered INVESTIGATIONAL. CGP and the use of circulating tumor DNA is considered INVESTIGATIONAL for all other indications. 1 The use of circulating tumor cells is considered INVESTIGATIONAL for all indications. -

1 What Is Pathology? James C
1 What is pathology? James C. E. Underwood History of pathology 4 Making diagnoses 9 Morbid anatomy 4 Diagnostic pathology 9 Microscopic and cellular pathology 4 Autopsies 9 Molecular pathology 5 Pathology, patients and populations 9 Cellular and molecular alterations in disease 5 Causes and agents of disease 9 Scope of pathology 5 The health of a nation 9 Clinical pathology 5 Preventing disability and premature death 9 Techniques of pathology 5 Pathology and personalised medicine 10 Learning pathology 7 Disease mechanisms 7 Systematic pathology 7 Building knowledge and understanding 8 Pathology in the problem-oriented integrated medical curriculum 8 3 PatHOLOGY, PatIENTS AND POPULatIONS 1 Keywords disease diagnosis pathology history 3.e1 1 WHat IS patHOLOGY? Of all the clinical disciplines, pathology is the one that most Table 1.1 Historical relationship between the hypothetic directly reflects the demystification of the human body that has causes of disease and the dependence on techniques for made medicine so effective and so humane. It expresses the truth their elucidation underpinning scientific medicine, the inhuman truth of the human body, and disperses the mist of evasion that characterises folk Techniques medicine and everyday thinking about sickness and health. Hypothetical supporting causal From: Hippocratic Oaths by Raymond Tallis cause of disease hypothesis Period Animism None Primitive, although Pathology is the scientific study of disease. Pathology the ideas persist in comprises scientific knowledge and diagnostic methods some cultures essential, first, for understanding diseases and their causes and, second, for their effective prevention and treatment. Magic None Primitive, although Pathology embraces the functional and structural changes the ideas persist in in disease, from the molecular level to the effects on the some cultures individual patient, and is continually developing as new research illuminates our knowledge of disease. -

The Molecular Pathology of Cutaneous Melanoma
Cancer Biomarkers 9 (2011) 267–286 267 DOI 10.3233/CBM-2011-0164 IOS Press The molecular pathology of cutaneous melanoma Thomas Bogenriedera and Meenhard Herlynb,∗ aBoehringer Ingelheim RCV, Dr. Boehringer Gasse 5-11, 1121 Vienna, Austria bThe Wistar Institute, 3601 Spruce Street, Philadelphia, PA, USA Abstract. Cutaneous melanoma is a highly aggressive cancer with still limited, but increasingly efficacious, standard treatment options. Recent preclinical and clinical findings support the notion that cutaneous melanoma is not one malignant disorder but rather a family of distinct molecular diseases. Incorporation of genetic signatures into the conventional histopathological classification of melanoma already has great implications for the management of cutaneous melanoma. Herein, we review our rapidly growing understanding of the molecular biology of cutaneous melanoma, including the pathogenic roles of the mitogen- associated protein kinase (MAPK) pathway, the phosphatidylinositol 3 kinase [PI3K]/phosphatase and tensin homologue deleted on chromosome 10 [PTEN]/Akt/mammalian target of rapamycin [mTOR])PTEN (phosphatase and tensin homolog) pathway, MET (hepatocyte growth factor), Notch signaling, and other key molecules regulating cell cycle progression and apoptosis. The mutation Val600Glu in the BRAF oncogene (designated BRAF(V600E)) has been associated with clinical benefit from agents that inhibit BRAF(V600E) or MEK (a kinase in the MAPK pathway). Cutaneous melanomas arising from mucosal, acral, chronically sun-damaged surfaces sometimes have oncogenic mutations in KIT, against which several inhibitors have shown clinical efficacy. These findings suggest that prospective genotyping of patients with melanoma, combined with the growing availability of targeted agents, which can be used to rationally exploit these findings, should be used increasingly as we work to develop new and more effective treatments for this devastating disease. -
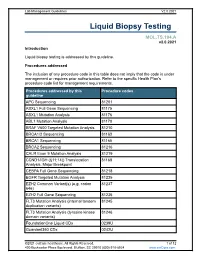
Liquid Biopsy Testing
Lab Management Guidelines V2.0.2021 Liquid Biopsy Testing MOL.TS.194.A v2.0.2021 Introduction Liquid biopsy testing is addressed by this guideline. Procedures addressed The inclusion of any procedure code in this table does not imply that the code is under management or requires prior authorization. Refer to the specific Health Plan's procedure code list for management requirements. Procedures addressed by this Procedure codes guideline APC Sequencing 81201 ASXL1 Full Gene Sequencing 81175 ASXL1 Mutation Analysis 81176 ABL1 Mutation Analysis 81170 BRAF V600 Targeted Mutation Analysis 81210 BRCA1/2 Sequencing 81163 BRCA1 Sequencing 81165 BRCA2 Sequencing 81216 CALR Exon 9 Mutation Analysis 81219 CCND1/IGH (t(11;14)) Translocation 81168 Analysis, Major Breakpoint CEBPA Full Gene Sequencing 81218 EGFR Targeted Mutation Analysis 81235 EZH2 Common Variant(s) (e.g. codon 81237 646) EZH2 Full Gene Sequencing 81236 FLT3 Mutation Analysis (internal tandem 81245 duplication variants) FLT3 Mutation Analysis (tyrosine kinase 81246 domain variants) FoundationOne Liquid CDx 0239U Guardant360 CDx 0242U ©2021 eviCore healthcare. All Rights Reserved. 1 of 12 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Lab Management Guidelines V2.0.2021 Procedures addressed by this Procedure codes guideline Hematolymphoid Neoplasm Molecular 81450 Profiling; 5-50 genes IDH1 Mutation Analysis 81120 IDH2 Mutation Analysis 81121 IGH@/BCL2 (t(14;18)) Translocation 81278 Analysis, Major Breakpoint Region (MBR) and Minor Cluster Region (mcr) Breakpoints JAK2 Targeted Mutation Analysis (e.g 81279 exons 12 and 13) JAK2 V617F Targeted Mutation Analysis 81270 KIT Targeted Sequence Analysis 81272 KIT D816 Targeted Mutation Analysis 81273 KRAS Exon 2 Targeted Mutation Analysis 81275 KRAS Targeted Mutation Analysis, 81276 Additional Variants MGMT Promoter Methylation Analysis 81287 MLH1 Sequencing 81292 Molecular Tumor Marker Test 81400 81401 81402 g 81403 n i 81405 t 81406 s e T 81407 81408 y s 81479 p o Molecular Tumor Marker Test 88271 i B MPL Common Variants (e.g. -

Promises and Pitfalls of Using Liquid Biopsy for Precision Medicine Giovanna Rossi1 and Michail Ignatiadis2
Published OnlineFirst May 20, 2019; DOI: 10.1158/0008-5472.CAN-18-3402 Cancer Review Research Promises and Pitfalls of Using Liquid Biopsy for Precision Medicine Giovanna Rossi1 and Michail Ignatiadis2 Abstract New sensitive assays are currently available for the detec- Multiple studies are underway to assess the clinical utility of tion of circulating tumor DNA (ctDNA) and circulating tumor CTC and ctDNA in different settings (treatment-na€ve vs. cells (CTC). However, there remains a need for standardiza- resistant, adjuvant vs. metastatic) and for different treatment tion of preanalytical issues and cross-platform comparison modalities (systemic therapy, surgery, radiation therapy). studies. Liquid biopsies are being evaluated for treatment This review aims to map the challenges that remain to be selection, for monitoring disease response and resistance, for addressed before liquid biopsies can be widely used for cancer tracking minimal residual disease, and for cancer diagnosis. management. Introduction applications of CTCs and ctDNA that are currently being explored are summarized in Fig. 1A. In the era of precision medicine, liquid biopsies are increasingly being studied as a tool that can capture tumor evolution in real time and thus guide systemic treatment. In this article, we will refer to the analysis of circulating tumor DNA (ctDNA) and circulating Challenges Associated with Preanalytical tumor cells (CTC) only and we will not cover other liquid biopsy Issues and the Analytical Validity of Liquid biomarkers such as circulating RNAs, proteins, metabolites, and Biopsy Assays exosomes. For CTC and ctDNA assays, there is a need to standardize Sampling a patient's blood may give information about the preanalytical variables and for cross-platform comparison stud- genomic profile of a given cancer (1–3) and provide an assess- ies. -

Overview of the AMA Molecular Pathology CPT Codes and Reimbursement
Overview of the AMA Molecular Pathology CPT codes and Reimbursement V.M. Pratt, PhD, FACMG Indiana University School of Medicine PrecisionPrecision Medicine Medicine Conference Conference Clinical Laboratory Testing and Reimbursement in Pharmacogenetics V.M. Pratt, PhD, FACMG Indiana University School of Medicine PrecisionPrecision Medicine Medicine Conference Conference Disclosure • I declare no conflicts of interest, real or apparent, and no financial interests in any company, product, or service mentioned in this program, including grants, employment, gifts, stock holdings, and honoraria. • Note: I am a member of AMA Molecular Pathology Workgroup, AMA Propriety Laboratory Assay Technical Advisory Group, and Center for Medicare and Medicaid Services, Advisory Panel Member on Clinical Diagnostic Laboratory Tests (all are voluntary positions) • The University of Florida College of Pharmacy is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. 3 Objectives • Describe laboratory testing and reimbursement models for pharmacogenetic testing. • Compare and contrast various strategies and methods for pharmacogenetic testing and reimbursement in clinical practice • Summarize reimbursement challenges in precision medicine and strategies for overcoming these challenges. • Determine appropriate use of CPT coding for pharmacogenetic testing 4 Reimbursement and CPT codes • CPT code ≠ reimbursement • List of services CPT is a registered trademark of the American Medical Association. ©2013 -

Original Article Retaining Antigenicity and DNA in the Melanin Bleaching of Melanin-Containing Tissues
Int J Clin Exp Pathol 2020;13(8):2027-2034 www.ijcep.com /ISSN:1936-2625/IJCEP0114621 Original Article Retaining antigenicity and DNA in the melanin bleaching of melanin-containing tissues Liwen Hu1, Yaqi Gao2, Caihong Ren1, Yupeng Chen1, Shanshan Cai1, Baobin Xie1, Sheng Zhang1, Xingfu Wang1 1Department of Pathology, Quality Control, The First Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian Province, China; 2Department of Quality Control, The First Affiliated Hospital of Fujian Medical University, China Received May 19, 2020; Accepted June 29, 2020; Epub August 1, 2020; Published August 15, 2020 Abstract: Preserving the antigen effectiveness and DNA when bleaching melanin from melanin-containing tissues is an important part of medical diagnosis. Some prior studies focused excessively on the speed of bleaching neglect- ing the preservation of antigen and DNA, especially the nucleic acids in the long-archived tissues. The approach of this study was to determine the optimal bleaching conditions by increasing the H2O2 concentration and to compare that with the high temperature and potassium-permanganate bleaching methods. The comparisons involve im- munohistochemical staining, HE staining, and gel electrophoresis, and setting the blank control (tissues without bleaching). The results demonstrated that bleaching using strong oxidizers or at high temperatures destroyed the antigen and DNA. Incubation with 30% H2O2 for 12 h at 24°C leaves only a small amount of melanin, preserving both the antigen effectiveness and the quality of the nucleic acids, and the target bands are clearly visible after PCR amplification. In conclusion, bleaching by increasing the concentration is a simple method, and it satisfies the requirements of clinical pathology and molecular pathology for the diagnosis and differential diagnosis of melanin- containing tissues. -

Molecular Pathological Epidemiology Gives Clues to Paradoxical Findings
Molecular Pathological Epidemiology Gives Clues to Paradoxical Findings The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Nishihara, Reiko, Tyler J. VanderWeele, Kenji Shibuya, Murray A. Mittleman, Molin Wang, Alison E. Field, Edward Giovannucci, Paul Lochhead, and Shuji Ogino. 2015. “Molecular Pathological Epidemiology Gives Clues to Paradoxical Findings.” European Journal of Epidemiology 30 (10): 1129–35. https://doi.org/10.1007/ s10654-015-0088-4. Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:41392032 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Open Access Policy Articles, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#OAP HHS Public Access Author manuscript Author Manuscript Author ManuscriptEur J Epidemiol Author Manuscript. Author Author Manuscript manuscript; available in PMC 2016 October 07. Published in final edited form as: Eur J Epidemiol. 2015 October ; 30(10): 1129–1135. doi:10.1007/s10654-015-0088-4. Molecular Pathological Epidemiology Gives Clues to Paradoxical Findings Reiko Nishiharaa,b,c, Tyler J. VanderWeeled,e, Kenji Shibuyac, Murray A. Mittlemand,f, Molin Wangd,e,g, Alison E. Fieldd,g,h,i, Edward Giovannuccia,d,g, Paul Lochheadi,j, and Shuji Oginob,d,k aDepartment of Nutrition, Harvard T.H. Chan School of Public Health, 655 Huntington Ave., Boston, Massachusetts 02115 USA bDepartment of Medical Oncology, Dana-Farber Cancer Institute and Harvard Medical School, 450 Brookline Ave., Boston, Massachusetts 02215 USA cDepartment of Global Health Policy, Graduate School of Medicine, The University of Tokyo, 7-3-1, Hongo, Bunkyo-ku, Tokyo, Japan dDepartment of Epidemiology, Harvard T.H. -

Stratification of HPV-Induced Cervical Pathology Using the Virally Encoded
Modern Pathology (2015) 28, 977–993 © 2015 USCAP, Inc All rights reserved 0893-3952/15 $32.00 977 Stratification of HPV-induced cervical pathology using the virally encoded molecular marker E4 in combination with p16 or MCM Heather Griffin1,2, Yasmina Soneji2, Romy Van Baars3, Rupali Arora4, David Jenkins3, Miekel van de Sandt3, Zhonglin Wu2, Wim Quint3, Robert Jach5, Krzysztof Okon5, Hubert Huras5, Albert Singer4 and John Doorbar1,2 1Department of Pathology, University of Cambridge, Cambridge, UK; 2National Institute for Medical Research, London, UK; 3DDL Diagnostic Laboratory, Rijswijk, The Netherlands; 4University College Hospital, London, UK and 5Department of Gynecology and Oncology, Jagiellonian University College, Krakow, Poland High-risk human papillomavirus (HPV) types cause cervical lesions of varying severity, ranging from transient productive infections to high-grade neoplasia. Disease stratification requires the examination of lesional pathology, and possibly also the detection of biomarkers. P16INK4a and MCM are established surrogates of high- risk HPV E6/E7 activity, and can be extensively expressed in high-grade lesions. Here we have combined these two cellular biomarkers with detection of the abundant HPV-encoded E4 protein in order to identify both productive and transforming lesions. This approach has allowed us to distinguish true papillomavirus infections from similar pathologies, and has allowed us to divide the heterogeneous CIN2 category into those that are CIN1- like and express E4, and those that more closely resemble nonproductive CIN3. To achieve this, 530 lesional areas were evaluated according to standard pathology criteria and by using a multiple staining approach that allows us to superimpose biomarker patterns either singly or in combination onto an annotated hematoxylin and eosin (H&E) image. -

Utility of Circulating Tumor DNA in Different Clinical Scenarios of Breast Cancer
cancers Review Utility of Circulating Tumor DNA in Different Clinical Scenarios of Breast Cancer Alexandra Mesquita 1,2,3,*, José Luís Costa 2,3 and Fernando Schmitt 2,3 1 Medical Oncology Department, Hospital Pedro Hispano, Unidade Local Saúde Matosinhos, 4464-513 Senhora da Hora, Portugal 2 Institute of Molecular Pathology and Immunology, University of Porto, 4200-135 Porto, Portugal; [email protected] (J.L.C.); [email protected] (F.S.) 3 Faculty of Medicine, University of Porto, 4200-319 Porto, Portugal * Correspondence: [email protected] Received: 3 November 2020; Accepted: 14 December 2020; Published: 16 December 2020 Simple Summary: This review is focused on the concept of a specific type of “liquid biopsy”, circulating cell-free tumor DNA (ctDNA). It explores the advantages and limitations of using this technique and the latest advances of using it in different clinical scenarios of breast cancer: early, metastatic, and locally advanced disease. It provides the latest advances in this area applied to clinical research and clinical practice, as well as the importance of the collaboration between clinicians and laboratory teams to fully grasp the potential of ctDNA in a precision medicine era. Abstract: Breast cancer is a complex disease whose molecular mechanisms are not completely understood. Developing target therapies is a promising approach. Therefore, understanding the biological behavior of the tumor is a challenge. Tissue biopsy in the metastatic setting remains the standard method for diagnosis. Nevertheless, it has been associated with some disadvantages: It is an invasive procedure, it may not represent tumor heterogeneity, and it does not allow for treatment efficacy to be assessed or early recurrences to be detected. -

Accelerating the Future of Liquid Biopsy
Accelerating the future of liquid biopsy Ultra-low mutation detection solutions from sample prep to data analysis The exciting potential of liquid biopsy in oncology research Currently, the most common strategy for characterizing the Recent studies have shown the utility of liquid biopsies for: genetic makeup of a tumor is the extraction, or biopsy, of a sample of the affected tissue. Tissue biopsies, however, • Enhancing understanding of tumorigenesis, metastasis, can be painful, risky, and in some cases not feasible when and therapy resistance a tumor is difficult to access. Furthermore, tissue biopsies • Detection of cancer at early stages when treatment may are not a viable monitoring technique as they cannot be be most successful repeated, and they may not be representative of the entire tumor due to tumor heterogeneity. • Monitoring of cancer development, disease progression, and recurrence Liquid biopsy is an emerging area of clinical research, • Tracking response or resistance during and after particularly in the context of cancer. As a minimally invasive treatment to allow for adjustments in real time complementary or alternative approach to tissue biopsies, liquid biopsies are less risky, painful, and costly, and are increasingly being used to analyze biomarkers in liquid samples, such as blood. 2 Unlock the potential in your liquid biopsy samples MagMAX cell-free nucleic acid isolation kits Liquid biopsies most often utilize cell-free DNA (cfDNA) that is derived from both normal and cancerous cells. The tumor-only supply of DNA in the bloodstream is more commonly referred to as circulating tumor DNA (ctDNA), which is loaded with information about a tumor that would otherwise be difficult to access.