Apoptosis in Developmental and Repair-Related Human Tooth Remodeling: a View from the Inside
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Ono -- PTH-Pthrp Receptor Signaling in Osterix-Expressing Progenitors.Pdf
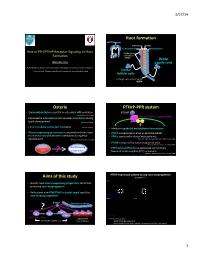
3/27/14 Root forma)on Cementum Dentin Cementoblast Odontoblast Role of PTH/PTHrP Receptor Signaling on Root Epithelial rests Formaon of Malassez (ERM) Dental Wanida Ono papilla cells AGE Orthodon;cs, Department of Developmental Biology, Harvard School of Dental Medicine Endocrine Unit, MassachuseLs General Hospital and Harvard Medical School Dental follicle cells Hertwig’s epithelial root sheath (HERS) Osterix PTHrP-PPR system • Transcripon factor essen;al to osteoblast differen;aon PTHrP (Nakashima K et al. 2002) PTH/PTHrP receptor (PPR) • Expressed in odontoblasts and alveolar osteoblasts during Gαs Gq tooth development (Chen S et al. 2009) • Controls cellular cementum formaon (Cao Z et al. 2012) • Mediates epithelial-mesenchymal interacons • Osterix-expressing precursors in the perichondrium move • PTHrP is expressed in enamel epithelia/HERS? to bone marrow and become osteoblasts during fetal • PPR is expressed in dental mesenchymes development (Maes C, Kronenberg HM et al. 2010) (Beck et al 1995; Lee Deeds and Segre 1995; Liu et al 1998) • PTHrP is required for tooth erup;on in mice (Philbrick WM, Karaplis AC et al. PNAS 1998) Osterix+ Root-forming • PPR haploinsufficiency is associated with primary cells progenitors failure of tooth erup;on (PFE) in humans ? (Decker E, Weber BH et al. Am J Hum Gen 2008) PTHrP expression paern during root morphogenesis Aims of this study PTHrPLacZ/+ x40 P7 P14 P49 • Iden;fy how osterix-expressing progenitors contribute to murine root morphogenesis • Understand how PTH/PTHrP receptor signal regulates root-forming progenitors PTHrP-LacZ x200 P7 x400 P14 ? PTHrP Epithelial root sheath PPR PTHrP-LacZ Osx+ progenitors Odontoblast PTHrP par;cipates in ……. -

Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes
Cells and Materials Volume 3 Number 2 Article 8 1993 Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes H. Lesot Institut de Biologie Médicale C. Begue-Kirn Institut de Biologie Médicale M. D. Kubler Institut de Biologie Médicale J. M. Meyer Institut de Biologie Médicale A. J. Smith Dental School, Birmingham See next page for additional authors Follow this and additional works at: https://digitalcommons.usu.edu/cellsandmaterials Part of the Biomedical Engineering and Bioengineering Commons Recommended Citation Lesot, H.; Begue-Kirn, C.; Kubler, M. D.; Meyer, J. M.; Smith, A. J.; Cassidy, N.; and Ruch, J. V. (1993) "Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes," Cells and Materials: Vol. 3 : No. 2 , Article 8. Available at: https://digitalcommons.usu.edu/cellsandmaterials/vol3/iss2/8 This Article is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Cells and Materials by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes Authors H. Lesot, C. Begue-Kirn, M. D. Kubler, J. M. Meyer, A. J. Smith, N. Cassidy, and J. V. Ruch This article is available in Cells and Materials: https://digitalcommons.usu.edu/cellsandmaterials/vol3/iss2/8 Cells and Materials, Vol. 3, No. 2, 1993 (Pages201-217) 1051-6794/93$5. 00 +. 00 Scanning Microscopy International, Chicago (AMF O'Hare), IL 60666 USA EXPERIMENTAL INDUCTION OF ODONTOBLAST DIFFERENTIATION AND STIMULATION DURING REPARATIVE PROCESSES 1 1 1 2 2 1 H. -

Journal of Dental Research
Journal of Dental Research http://jdr.sagepub.com/ Cell Differentiation and Matrix Organization in Engineered Teeth A. Nait Lechguer, M.L. Couble, N. Labert, S. Kuchler-Bopp, L. Keller, H. Magloire, F. Bleicher and H. Lesot J DENT RES 2011 90: 583 originally published online 4 February 2011 DOI: 10.1177/0022034510391796 The online version of this article can be found at: http://jdr.sagepub.com/content/90/5/583 Published by: http://www.sagepublications.com On behalf of: International and American Associations for Dental Research Additional services and information for Journal of Dental Research can be found at: Email Alerts: http://jdr.sagepub.com/cgi/alerts Subscriptions: http://jdr.sagepub.com/subscriptions Reprints: http://www.sagepub.com/journalsReprints.nav Permissions: http://www.sagepub.com/journalsPermissions.nav >> Version of Record - Apr 13, 2011 OnlineFirst Version of Record - Feb 4, 2011 What is This? Downloaded from jdr.sagepub.com at Service Commun de la Documentation Université de Strasbourg on September 6, 2013 For personal use only. No other uses without permission. © 2011 International & American Associations for Dental Research RESEARCH REPORTS Biomaterials & Bioengineering A. Nait Lechguer1,2, M.L. Couble3,4, N. Labert3,4, S. Kuchler-Bopp1,2, Cell Differentiation and L. Keller1,2, H. Magloire3,4, F. Bleicher3,4, Matrix Organization in and H. Lesot1,2* Engineered Teeth 1INSERM UMR 977, Faculté de Médecine, 11, rue Humann, F-67085 Strasbourg, France; 2Dental School, University of Strasbourg, Strasbourg, France; 3Université de Lyon, Faculté d’Odontologie, Rue Guillaume Paradin, F-69372 Lyon Cedex 08, France; and 4IGFL, CNRS UMR 5242, Ecole Normale Supérieure, 46 Allée d’Italie, 69364, Lyon Cedex 08, France; *corresponding author, [email protected] J Dent Res 90(5):583-589, 2011 ABSTRACT InTRODuCTIOn Embryonic dental cells were used to check a series of criteria to be achieved for tooth engineering. -

Dental Cementum Reviewed: Development, Structure, Composition, Regeneration and Potential Functions
Braz J Oral Sci. January/March 2005 - Vol.4 - Number 12 Dental cementum reviewed: development, structure, composition, regeneration and potential functions Patricia Furtado Gonçalves 1 Enilson Antonio Sallum 1 Abstract Antonio Wilson Sallum 1 This article reviews developmental and structural characteristics of Márcio Zaffalon Casati 1 cementum, a unique avascular mineralized tissue covering the root Sérgio de Toledo 1 surface that forms the interface between root dentin and periodontal Francisco Humberto Nociti Junior 1 ligament. Besides describing the types of cementum and 1 Dept. of Prosthodontics and Periodontics, cementogenesis, attention is given to recent advances in scientific Division of Periodontics, School of Dentistry understanding of the molecular and cellular aspects of the formation at Piracicaba - UNICAMP, Piracicaba, São and regeneration of cementum. The understanding of the mechanisms Paulo, Brazil. involved in the dynamic of this tissue should allow for the development of new treatment strategies concerning the approach of the root surface affected by periodontal disease and periodontal regeneration techniques. Received for publication: October 01, 2004 Key Words: Accepted: December 17, 2004 dental cementum, review Correspondence to: Francisco H. Nociti Jr. Av. Limeira 901 - Caixa Postal: 052 - CEP: 13414-903 - Piracicaba - S.P. - Brazil Tel: ++ 55 19 34125298 Fax: ++ 55 19 3412 5218 E-mail: [email protected] 651 Braz J Oral Sci. 4(12): 651-658 Dental cementum reviewed: development, structure, composition, regeneration and potential functions Introduction junction (Figure 1). The areas and location of acellular Cementum is an avascular mineralized tissue covering the afibrillar cementum vary from tooth to tooth and along the entire root surface. Due to its intermediary position, forming cementoenamel junction of the same tooth6-9. -

Basic Histology (23 Questions): Oral Histology (16 Questions
Board Question Breakdown (Anatomic Sciences section) The Anatomic Sciences portion of part I of the Dental Board exams consists of 100 test items. They are broken up into the following distribution: Gross Anatomy (50 questions): Head - 28 questions broken down in this fashion: - Oral cavity - 6 questions - Extraoral structures - 12 questions - Osteology - 6 questions - TMJ and muscles of mastication - 4 questions Neck - 5 questions Upper Limb - 3 questions Thoracic cavity - 5 questions Abdominopelvic cavity - 2 questions Neuroanatomy (CNS, ANS +) - 7 questions Basic Histology (23 questions): Ultrastructure (cell organelles) - 4 questions Basic tissues - 4 questions Bone, cartilage & joints - 3 questions Lymphatic & circulatory systems - 3 questions Endocrine system - 2 questions Respiratory system - 1 question Gastrointestinal system - 3 questions Genitouirinary systems - (reproductive & urinary) 2 questions Integument - 1 question Oral Histology (16 questions): Tooth & supporting structures - 9 questions Soft oral tissues (including dentin) - 5 questions Temporomandibular joint - 2 questions Developmental Biology (11 questions): Osteogenesis (bone formation) - 2 questions Tooth development, eruption & movement - 4 questions General embryology - 2 questions 2 National Board Part 1: Review questions for histology/oral histology (Answers follow at the end) 1. Normally most of the circulating white blood cells are a. basophilic leukocytes b. monocytes c. lymphocytes d. eosinophilic leukocytes e. neutrophilic leukocytes 2. Blood platelets are products of a. osteoclasts b. basophils c. red blood cells d. plasma cells e. megakaryocytes 3. Bacteria are frequently ingested by a. neutrophilic leukocytes b. basophilic leukocytes c. mast cells d. small lymphocytes e. fibrocytes 4. It is believed that worn out red cells are normally destroyed in the spleen by a. neutrophils b. -

The Role of Transforming Growth Factor Beta in Tertiary Dentinogenesis
The role of transforming growth factor beta in tertiary dentinogenesis Tetiana Haniastuti1, Phides Nunez2, and Ariadna A. Djais3 1Department of Oral Biology, Gadjah Mada University, Indonesia 2Division of Oral Ecology in Health and Infection, Niigata University, Japan 3Department of Oral Biology, University of Indonesia, Indonesia ABSTRACT The most visible repair response to pulp injury is the deposition of a tertiary dentin matrix over the dentinal tubules of the primary or secondary dentin. Tertiary dentin is distinguished as reactionary and reparative dentin, depending on the severity of the initiating response and the conditions under which the newly deposited dentin matrix was elaborated. Transforming growth factor beta (TGF-b) superfamily is a large group of growth factors that serve important roles in regulating cell growth, differentiation, and function. Members of this superfamily have been implicated in the repair process of the dental tissue after injury. Although numerous studies have proved that those bioactive molecules carry out an important role in the formation of tertiary dentin, comprehensive report regarding that phenomenon is not yet available. This review article aimed to summarize the role of TGF-b on tertiary dentinogenesis during the progression of a carious lesion. Key words: transforming growth factor beta, tertiary dentinogenesis, reactionary dentin, reparative dentin Correspondence: Tetiana Haniastuti, c/o: Bagian Biologi Oral, Fakultas Kedokteran Gigi Universitas Gadjah Mada. Jln. Denta no. 1, Sekip Utara Yogyakarta 55281, Indonesia. E-mail: [email protected] INTRODUCTION such as cell proliferation, differentiation, and matrix synthesis. Recent studies showed that this substance has a The complex structural composition of teeth provides significant role in the immune response7 and tissue repair8 hardness and durability as a barrier against bacterial of the dental pulp. -

Role of Connexin 43 in Odontoblastic Differentiation and Structural Maintenance in Pulp Damage Repair
International Journal of Oral Science www.nature.com/ijos ARTICLE OPEN Role of connexin 43 in odontoblastic differentiation and structural maintenance in pulp damage repair Jiaxin Yin1,2, Jue Xu3, Ran Cheng1, Meiying Shao3, Yuandong Qin1, Hui Yang1 and Tao Hu1 Dental pulp can initiate its damage repair after an injury of the pulp–dentin complex by rearrangement of odontoblasts and formation of newly differentiated odontoblast-like cells. Connexin 43 (Cx43) is one of the gap junction proteins that participates in multiple tissue repair processes. However, the role of Cx43 in the repair of the dental pulp remains unclear. This study aimed to determine the function of Cx43 in the odontoblast arrangement patterns and odontoblastic differentiation. Human teeth for in vitro experiments were acquired, and a pulp injury model in Sprague-Dawley rats was used for in vivo analysis. The odontoblast arrangement pattern and the expression of Cx43 and dentin sialophosphoprotein (DSPP) were assessed. To investigate the function of Cx43 in odontoblastic differentiation, we overexpressed or inhibited Cx43. The results indicated that polarized odontoblasts were arranged along the pulp–dentin interface and had high levels of Cx43 expression in the healthy teeth; however, the odontoblast arrangement pattern was slightly changed concomitant to an increase in the Cx43 expression in the carious teeth. Regularly arranged odontoblast-like cells had high levels of the Cx43 expression during the formation of mature dentin, but the odontoblast- like cells were not regularly arranged beneath immature osteodentin in the pulp injury models. Subsequent in vitro experiments demonstrated that Cx43 is upregulated during odontoblastic differentiation of the dental pulp cells, and inhibition or overexpression of Cx43 influence the odontoblastic differentiation. -

Developmental Biology of Cementum
Int. J. Dev. Biol. 45: 695-706 (2001) Review Developmental Biology of Cementum THOMAS G.H. DIEKWISCH* Allan G. Brodie Laboratory for Craniofacial Genetics, University of Illinois at Chicago, USA CONTENTS Origins of cementum - a scientific "whodunit" ........................................................................695 Loss of ameloblast continuity and insertion of mesenchymal cells from the dental follicle proper ................................................................................................697 Initial cementum matrix deposition by mesenchymal cells in proximity to non-secretory epithelial cells ...................................................................................699 Cementogenesis at the tooth cervix and at the cemento-enamel junction .............................700 Early removal of HERS from the root surface in humans as seen in the Gottlieb collection ..............................................................................................701 Role of amelogenins in cementogenesis ................................................................................702 Possible mechanism of cementoblast induction .....................................................................704 Summary ................................................................................................................................704 KEY WORDS: Cementum, Hertwig’s epithelial root sheath, Gottlieb, amelogenin, periodontium Tooth cementum is a bone-like mineralized tissue secreted by Origins of cementum - a scientific -

Initiation to Eruption
Head and Neck embryology Tooth Development Review head and neckblk embryology Initiation to eruption Skip Review Initiation Initiation stomodeum Epithelial cells (dental lamina) During 6th week, ectoderm in stomodeum forms horseshoe shaped mass of oral epithelium mesenchyme Basement membrane mesenchyme Initiation of anterior primary teeth Epithelial cells in horseshoe Dental lamina begins begins the sixth to seventh week form dental lamina growing into mesenchyme of development, initiation of additional At site where tooth will be teeth follows and continues for years Dental Lamina – Initiation Supernumerary tooth PREDICT what would happen if an extra tooth was initiated. Mesiodens 1 Bud Stage – eighth week Bud Stage Epithelium (dental Lamina) Dental lamina grows down into mesenchyme at site of tooth. Mesenchyme starts to change composition in response mesenchyme PREDICT what would happen if two tooth buds fused together or one tooth bud split in half. Fusion/Gemination Cap stage – week 9 By week 9, all germ layers of future tooth have formed ElEnamel organ (ename ll)l only) Dental papilla (dentin and pulp) Fusion Gemination Dental sac (cementum, PDL, Alveolar bone) PREDICT how you would know if it was mesenchyme fusion or gemination Cap Stage Successional Dental Lamina Each primary tooth germ has epithelium a successional lamina that becomes a permanent tooth Succedaneous teeth replace a deciduous tooth, nonsuccedaneous do not IDENTIFY nonsuccedaneous teeth mesenchyme PREDICT What occurs if no successional lamina forms? 2 Congenitally -

Shh and Epithelial Growth and Polarity
Development 129, 5323-5337 5323 © 2002 The Company of Biologists Ltd doi:10.1242/dev.00100 Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization Amel Gritli-Linde1,*, Marianna Bei2, Richard Maas2, Xiaoyan M. Zhang3, Anders Linde1 and Andrew P. McMahon4,* 1Department of Oral Biochemistry, Sahlgrenska Academy at Göteborg University, SE-405 30 Göteborg, Sweden 2Division of Genetics, Brigham and Women’s Hospital, Harvard Medical School, 20 Shattuck Street, Boston, MA 02115, USA 3Curis Inc., 45 Moulton Street, Cambridge, MA 02138, USA 4Department of Molecular and Cellular Biology, Harvard University, 16 Divinity Avenue, Cambridge, MA 02135, USA *Authors for correspondence (e-mail: [email protected] and [email protected]) Accepted 12 August 2002 SUMMARY Sonic hedgehog (Shh), a member of the mammalian epithelium should block Shh signaling within dental Hedgehog (Hh) family, plays a key role during epithelial derivatives while preserving normal embryogenesis and organogenesis. Tooth development, mesenchymal signaling. Here we show that Shh-dependent odontogenesis, is governed by sequential and reciprocal interactions occur within the dental epithelium itself. The epithelial-mesenchymal interactions. Genetic removal of dental mesenchyme develops normally up until birth. In Shh activity from the dental epithelium, the sole source of contrast, dental epithelial derivatives show altered Shh during tooth development, alters tooth growth and proliferation, growth, differentiation and polarization. -

1 Harmine Stimulates the Differentiation of Cementoblasts In
Harmine stimulates the differentiation of cementoblasts in vitro Thesis Presented in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Graduate School of The Ohio State University By Li Zheng, DDS, PhD The Ohio State University 2020 Thesis Committee Dr. Toru Deguchi, Advisor Dr. Brian L. Foster Dr. Do-Gyoon Kim 1 Copyrighted by Li Zheng 2020 2 Abstract Introduction: One of the major side effects in orthodontic treatment is external apical root resorption (EARR). Recently, a new antiresorptive agent, harmine, was reported to stimulate osteoblast formation, differentiation and function. We hypothesized that harmine can be a potential agent for preventing and even recovering EARR. Therefore, our objective is to examine if harmine stimulates the differentiation of cementoblast in vitro. Methods: A cementoblast cell line (OCCM-30) was obtained for growth assay and scratch assay to investigate the effects of harmine on cementoblast growth. Real-time Polymerase Chain Reaction (RT-PCR) was used to examine transcription factors and differentiation markers such as Runx2, Dlx5, ctnnb1, Msx2, Osterix, Ibsp, Col1a1 and Spp1 in cementoblasts treated with harmine, compared to cells without treatment. Western blot was used to test if the transcription factors and differentiation markers are stimulated or inhibited on the protein level. Differentiation assays such as alizarin red staining and von Kossa staining are used to examine the effects of harmine on cementoblast differentiation. Results: The growth rate of OCCM-30 was inhibited by 10 µm harmine after 2 days treatment and more significant on day 3. Furthermore, scratch assay study showed a dose-dependent inhibition manner. -

Odontoblast Differentiation
Int. J. Dev. BioI. 39: 51-68 (1995) 51 Special Review Odontoblast differentiation JEAN VICTOR RUCH*, HERVE LESOT and CATHERINE BEGUE-KIRN Institut de 8iologie Medica/e, INSERM U 424, Strasbourg, France CONTENTS InIrod uction .......................................................................................................................... 52 From neural crest to the initial bell stage: the black box 52 Tooth patterning 52 Generation of tooth cell diversity 54 Cytological and functional aspects of odontoblast terminal differentiation .................. 54 Epigenesis of odontoblast terminal differentiation 57 The inner dental epithelium controls odontoblast terminal differentiation 57 The dental basement membrane plays a role in odontoblast differentiation 57 Dental basement membrane changes accompany odontoblast differentiation 58 A stage- and space-specific basement membrane is involved in odontoblast differentiation 59 Growth factors and odontoblast differentiation: descriptive data 59 Aspects of cell proliferation kinetics: the problem of competence 59 Fibronectin plays a role in odontoblast polarization 61 Effects of matrix molecules on odontoblast differentiation in vitro 62 Effects of TGFI31, BMP2 and IGFI on odontoblast differentiation 62 Current hypotheses and questions 62 Conclusion 65 Summary and key words ..................................................................................................... 65 References ........ 65 'Addre.. for reprints: Institut de Biologie Medicale, Faculte de Medecine, 11, rue Humann,