CML Handbook of Chronic Myeloid Leukemia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SA Health Job Pack
SA Health Job Pack Data Entry, Call Centre, Courier - Casual Pool - OPS COVID 19 Job Title - SA Pathology Eligibility Open to Everyone Job Number 763086 Applications Closing Date 31/12/21 Region / Division Statewide Clinical Support Services Health Service SA Pathology Location Metropolitan and Regional positions Classification OPS-1 Job Status Casual opportunities Salary $14.53 - $26.73 per hour plus 25% casual leave loading Contact Details Full name Jill Horn Phone number 7117 2427 Email address [email protected] Criminal History Assessment Applicants will be required to demonstrate that they have undergone an appropriate criminal and relevant history screening assessment/ criminal history check. Depending on the role, this may be a Department of Communities and Social Inclusion (DCSI) Criminal History Check and/or a South Australian Police (SAPOL) National Police Check (NPC). The following checks will be required for this role: Working with Children Screening - DHS Vulnerable Person-Related Employment Screening - NPC Aged Care Sector Employment Screening - NPC General Employment Probity Check - NPC Further information is available on the SA Health careers website at www.sahealth.sa.gov.au/careers - see Career Information, or by referring to the nominated contact person below. For Official Use Only – I1-A1 [Template updated May 2018] Page 1 of 15 Immunisation Risk Category A (direct contact with blood or body substances) This role carries specific immunisation requirements. To be eligible for appointment in this role you will be required to meet the immunisation requirements associated with Category A (direct contact with blood or body substances). Please click here for further information on these requirements. -

Leukemoid Reaction in a Patient with Acute Lymphoblastic Leukemia Following the Second Chemotherapy
262JCEI / Yokuş et al. Leukemoid reaction in ALL 2013; 4 (2): 262-263 Journal of Clinical and Experimental Investigations doi: 10.5799/ahinjs.01.2013.02.0280 LETTER TO EDITOR Leukemoid reaction in a patient with acute lymphoblastic leukemia following the second chemotherapy Akut lenfoblastik lösemili hastada ikinci kemoterapi sonrası gelişen lökomoid reaksiyon Osman Yokuş1, Murat Albayrak2, Aynur Albayrak3, Habip Gedik4 To the Editor, to 50.000 cells/µL. The examined peripheral blood smear appeared as similar to the chronic phase of The occurrence of persistent neutrophilic leukocy- CML (fig.2). A cytogenetic examination of Philadel- tosis above 50,000 cells/µL for reasons other than phia chromosome [t(9:22] was found to be nega- leukemia is defined as leukemoid reaction. Chronic tive, therefore, CML was excluded in the patient. myelogenous leukemia (CML) and chronic neutro- Hydroxurea was initiated once a day. Leukocytosis philic leukemia (CNL) should be excluded, and un- recovered to normal range after one week (fig.3) derlying diseases or causes should be examined, and bone marrow examination revealed remission. in differential diagnosis. The most commonly ob- Cytogenetic analysis was normal and this leukocy- served causes of leukemoid reactions are severe tosis was determined as reactive leukemoid reac- infections, intoxications, malignancies, severe hem- tion. We aimed to report this case owing to the fact orrhage, or acute hemolysis [1]. J Clin Exp Invest that leukocytosis associated with G-CSF, which is 2013; 4 (2): 262-263 used to increase the leukocyte count during neutro- Rarely, leukemoid reaction can be seen in pa- penia, has been reported previously but it occurred tients with acute leukemia subsequent to chemo- after second chemotherapy without G-CSF [4]. -

Wikipedia: the Term Leukemoid Reaction Describes an Elevated White Blood
Correspondence: I. Potasman, MD Leukemoid Reaction: Spectrum and Prognosis of 173 Adult Patients Infectious Diseases, ABSTRACT No. Bnai Zion Med. Ctr. 47 Golomb St., Haifa 31048. 39147 Israel Potasman, MD and Moti Grupper, MD Tel. 972-48359055 Fax. 972-48359755 Bnai Zion Med. Ctr., Haifa, Israel Email: [email protected] LR- CLINICAL and LABORATORY FEATURES CHRONIC DISEASES & CONDITIONS CAUSING LR METHODS WikipediA: The term leukemoid reaction describes an elevated white blood cell count, or leukocytosis, that is a physiological response to stress or infection (as Table 1: Leukemoid Reaction: Demographics, Major Clinical, and Laboratory features, and Table 2: Chronic & possible Instigating diseases Causing Leukemoid Reaction (n=173) The BZMC is a 411 bed university hospital located in Haifa, Israel. Mortality (n=173) Disease/Drug Occurrences (%) opposed to a primary blood malignancy, such as leukemia). Contains all basic departments except for neurosurgery, cardiovascular surgery and solid-tumor oncology. The respi- Parameter N (%) Alive Dead n Age (years, mean ±S.D.) 69.4 ±19.6 63.3±21.2 79.4±11.2 Background ratory intensive care unit inhabits six patients; additional respirators are in use in the 3 departments of medicine. History of Cardiovascular 86 (49.7%) Minimal-Maximal 21-97 21-97 28-97 ABSTRACT During an average year there are 29,508 hospitalizations of adults (>18 years) with ~150,000 hospitalization-days, Median 75 69 81 disease Diabetes Mellitus 46 (26.6%) and an average occupancy of 92%. Patients were eligible throughout hospital stay, and wherever they were admit- Chronic Lung disease 29 (16.8%) ted. -
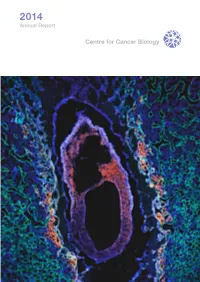
CCB Annual Report 2014
2014 Annual Report Centre for Cancer Biology 2014 Annual Report Centre for Cancer Biology 2 Organisation 3 Alliance Partners Report 4 Centre for Cancer Biology Directors Report 6 Celebrating Our Strategic Alliance Laboratories 8 Acute Leukaemia Laboratory 10 Cell Signalling Laboratory 12 Cytokine Receptor Laboratory 14 Drug Discovery and Development Laboratory 16 Gastroenterology Research Laboratory 18 Gene Regulation Laboratory 20 Hepatitis C Virus Research Laboratory 22 Leukaemia Unit, Genetics and Molecular Pathology 24 Lymphatic Development Laboratory 26 Mast Cell Laboratory 28 Molecular Pathology Research Laboratory 30 Molecular Regulation Laboratory Centre for Cancer Biology 32 Molecular Signalling Laboratory An alliance between 34 Myeloma Research Laboratory SA Pathology and the University of South Australia 36 Neurovascular Research Laboratory SA Pathology 38 Translational Oncology Laboratory Frome Road, Adelaide South Australia 5000 40 Tumour Microenvironment Laboratory Australia 42 Vascular Biology and Cell Trafficking Laboratory T +61 8 8222 3422 F +61 8 8232 4092 44 ACRF Cancer Genomics Facility General Enquiries Ms Anna Nitschke 46 Publications Executive Assistant to Professor Angel Lopez [email protected] 51 Financial Highlights Postal Address 52 New Grants and Fellowships PO Box 14 Rundle Mall Adelaide South Australia 5000 Publication Coordination 54 Seminar Program Australia Anna Nitschke Centre for Cancer Biology 56 Invited Presentations www.centreforcancerbiology.org.au Design and Production Catherine Buddle 58 Awards Buddle Design 60 Research Staff and Students cover image Photography Mark Fitz-Gerald and Peter Dent Sagittal section of an E7.0 embryo surrounded by yolk sac and endometrial tissue SA Pathology Photo & Imaging 62 Our Supporters immunostained to recognise Neuropilin 2 (red), E-cadherin (green) and DAPI (blue). -

Management of Hepatic Burkitt Lymphoma in Children
Gastroenterology & Hepatology International Journal ISSN: 2574-8009 Management of Hepatic Burkitt Lymphoma in Children Elhoudzi J* and Harif M Case Report Pediatric Hematology & Oncology Department, University Hospital Center, Morocco Volume 3 Issue 1 Medical school of Marrakesh, Cady Ayad University, Morocco Received Date: June 02, 2018 Published Date: June 15, 2018 *Corresponding author: Jamila Elhoudzi, Pediatric Hematology & Oncology Department, University Hospital Center, Morocco, Ibn Sina street, Ammerchich, 40000, Morocco, Tel: 00212661544256; Email: [email protected] . Abstract Hepatic Burkitt lymphoma is extremely rare in childhood and can be overlooked in differential diagnosis of liver masses. Patient: A8-year-old girl presented with a 1 month history of abdominal pain and weight loss and jaundice. Results: Physical examination revealed hepatomegaly and no palpable lymph node. Laboratory finding showed mild anemia (hemoglobin, 10,8 g/dL), elevated transaminase (ALT, 305 IU/L; ASAT,755 IU/L), elevated bilitubin (Bilirubin total, 179mg/L, Bilirubin direct, 143mg/l). Abdominal ultrasound shouwed a multiple hepatic lesions. Liver biopsy examination confirmed Burkitt's lymphoma. No metastasis was detected in the thoracic cavity, bone marrow, and spinal fluid. The patient was treated with the combination regimen of cyclophosphamide, doxorubicin, vincristine, prednisone and high dose methotrexate. Cytosine arabinoside and methotrexate were added for CNS prophylaxis by intrathecal installation. Serial follow-up ultrasound showed a marked decrease in the size of hepatic lesions but residual hilar lymph nodes at 2cm and the control showed stable size of lymph node after 28 months of chemotherapy. Conclusion: The clinical feature of primary hepaticlymphoma varies from no symptom to fulminant hepatic failure. There are no specific imaging criteria for diagnosing primary hepatic Burkitt’s lymphoma. -

Department for Health and Wellbeing 2019-20 Annual Report
OFFICIAL 2019-20 ANNUAL REPORT for the Department for Health and Wellbeing Department for Health and Wellbeing 2019-20 Annual Report Department for Health and Wellbeing PO Box 287 Rundle Mall Adelaide SA 5000 www.sahealth.sa.gov.au Contact phone number: +61 8 8226 0795 Contact email: [email protected] ISSN: 2201-0475 Date presented to Minister: 30 September 2020 1 | P a g e OFFICIAL OFFICIAL 2019-20 ANNUAL REPORT for the Department for Health and Wellbeing To: Hon Stephen Wade MLC Minister for Health and Wellbeing This annual report will be presented to Parliament to meet the statutory reporting requirements of the Public Sector Act 2009, the Public Sector Regulations 2010 and the Public Finance and Audit Act 1987 and the requirements of Premier and Cabinet Circular PC013 Annual Reporting. This report is verified to be accurate for the purposes of annual reporting to the Parliament of South Australia. Submitted on behalf of the Department for Health and Wellbeing by: Dr Christopher McGowan Chief Executive Date______________28-09-2020 Signature ________________ 2 | P a g e OFFICIAL OFFICIAL 2019-20 ANNUAL REPORT for the Department for Health and Wellbeing From the Chief Executive The Department for Health and Wellbeing (the department) as system leader, is committed to supporting the delivery of high quality public health services. Safety, equity, access and responsiveness of services are areas of focus for operations and service delivery to benefit the health and wellbeing of all South Australians. Across the year, the department has continued to improve whole-of-system capability and performance through partnership and connectivity. -

The University of Adelaide
RF: 2020/3866 South Australian Productivity Commission GPO Box 2343 ADELAIDE SA 5001 Email: [email protected] 8 May 2020 South Australian Productivity Commission Inquiry into Health and Medical Research in South Australia – submission from the University of Adelaide On behalf of the University of Adelaide, please find attached a detailed submission in response to the March 2020 SAPC Health and Medical Research Inquiry Issues Paper. As a major partner in the South Australian Health and Biomedical Precinct, the University is eager to participate in this initiative to identify opportunities to improve the State’s capability to attract investment in Health and Medical Research. Yours sincerely PROFESSOR ANTON PJ MIDDELBERG Deputy Vice-Chancellor and Vice-President (Research) Attachment: University of Adelaide submission to the SAPC Inquiry into Health and Medical Research in South Australia. PROFESSOR ANTON PJ MIDDELBERG DEPUTY VICE-CHANCELLOR AND VICE-PRESIDENT (RESEARCH) The University of Adelaide SA 5005 AUSTRALIA Tel: +61 8 8313 5665 Email: [email protected] www.adelaide.edu.au CRICOS provider number 00123M South Australian Productivity Commission Inquiry into Health and Medical Research in South Australia Submission from the University of Adelaide, 8 May 2020 ________________________________________________________________________________ This submission is presented on behalf of the University of Adelaide by Professor Anton Middelberg, Deputy Vice-Chancellor and Vice-President (Research). It is structured around the Inquiry terms of -

BHS Leucocytosis and Leucopenia Dr Caers
Leucocytoses & leucopenia Jo Caers Dept of Clinical Hematology [email protected] Bone marrow Blood Granulopoiesis Proliferation & 5 days maturation Storage 1 day Marginisation& Circulation 1 day Tissues 1-2 days Granulopoiesis Proliferation & 5 days maturation Storage 1 day Marginisation& Circulation 1 day Tissues 1-2 days Granulopoiesis Proliferation & 5 days maturation Storage 1 day Marginisation& Circulation 1 day Tissues 1-2 days Lymphocytes Monocytes NORMAL BLOOD CELL COUNT Hemoglobin 12.0 – 15.0 g/dl (F) 13.0 – 17.0 g/dl (M) Red Blood Cells 3.9 – 5.6 x 10 6/µl (F) 4.5 – 6.5 x 10 6/µl (M) Hematocrit 36 – 48% (F) 40 – 52% (M) Mean Corpuscular Volume 80 – 95µ³ Mean Hb Concentration 27 – 34 pg Conc corp mean Hb 30 – 35 g/dl Reticulocytes 0.5 – 20 % Leucocytes 4.0 – 10.0 x 10 3/µl ¨ Neutophils 1.8 – 7.5 Lymphocytes 1.5 – 3.5 Monocytes 0.2 – 0.8 Eosinophils 0.04 – 0.45 Basophils 0.01 – 0.1 Platelets 100 – 400 x 10 3/dl Hyperleucocytosis > 10.000 WBC/mm³ Normal Cells Abnormal cells or blastic cells Infections ? Neutrophilia (> 7.500/mm³) Acute Leukemias Lymphocytosis (> 4.500/mm³) Chronic Myelomonocytic Leukemias Chronic Lymhocytic leukemia Chronic Myeloid Leukemia Non Hodgkin Lymphoma Chronic Monocytosis (> 800/mm³) … Inflammations? Eosinophilia (> 400/mm³) Basophilia (> 100/mm³) Leukoerythroblastic reaction Cytopenia with immature RBCs (normoblasts) immature WBCs (agranular neutrophils, myelocytes, metamyelocytes ) Causes BM infiltration • Solid tumor or hematological malignancy • Myelofibrosis Strong BM stimulation • Infection, -

ITA-Biomed Bone and Joint-SA Meeting Adelaide South Australia 21-23 February 2011
Promoting opportunities for scientific and commercial collaboration in biomedical bone and joint research between Italy and South Australia 21-23 February 2011 Adelaide South Australia The University of Adelaide, Union House ITA-Biomed Bone and Joint-SA Meeting Adelaide, South Australia, 21-23 February 2011 The meeting organisers would like to acknowledge the support received from: Financial Support The TM Endorsed by From the chairman of the organising committee Dear delegates, Welcome to all the speakers that have accepted the invitation to attend this meeting. In particular welcome to our guests and speakers from Italy. I hope that all delegates will find their attendance a stimulating research experience and that it presents new research opportunities for collaboration with researchers in South Australia. The aim of this meeting is to promote bilateral scientific and commercial collaboration in biomedical bone and joint research between leading Italian, South Australian and Australian researchers and research organisations. As leaders of research groups covering areas of biomedical research focussing on bone and joint research, you have been invited to explore your combined research capabilities, through this face-to-face networking opportunity, to promote bilateral international collaboration. The meeting has been planned for a selected audience of about 25 participants, playing key roles in their area of research interest. Selected Italian research group leaders and scientists have been invited, along with selected research group leaders and scientists from South Australia and Australia (SA Pathology, Royal Adelaide Hospital, Women’s & Children’s Hospital, The University of Adelaide, Flinders University, University of South Australia, University of Sydney). We also have the participation of industry representatives from Xradia (Pleasanton, CA, USA), who will present to the audience the state of their R&D programs, and their vision or opportunities for collaboration. -

Reactive Splenomegaly and Variation of Lymphoid-Plasma Cell Population Associated with a Mouse Pituitary Thyrotropic Tumor*
Reactive Splenomegaly and Variation of Lymphoid-Plasma Cell Population Associated with a Mouse Pituitary Thyrotropic Tumor* EDITH E. SPROULANDRAUL GRINBERG (Rosiceli Park Memorial Institute and New York Slate Department of Health, Buffalo, New York) SUMMARY A transplantable thyrotropic pituitary tumor, subline L24a, which grew after 13 months of dormancy in an intact mouse was associated in its early passage with a variety of lymphoid tumors. On subsequent passage, slow growth over a 6- to 12- month period was regularly accompanied by striking splenomegaly without lymphoid or thymic neoplasia. The enlargement was due to a great increase in cells of the lymphoid series in the red pulp without loss of splenic architecture. In thyroidec- tomized mice plasma cells with many Russell bodies were especially numerous, and these cells were found hi hypertrophied lymphoid follicles within other organs. It is suggested that the splenomegaly and, hi earlier passage the lymphoid tumors, may represent an exaggerated antibody response to a tumor's having unusual or abnormal hormonal activity. The transplanted pituitary tumors, in turn, showed severe hemor- rhagic degeneration with peripheral small lymphocyte stasis in the lymphatics. Initial observations concerning pleomorphic reticulum through the courtesy of Dr. Jacob Furth, was studied cell- and lymphosarcomas or lymphoid hyperplasias through repeated passage for purposes of comparison. regularly accompanying a thyrotropic mouse pituitary This tumor has maintained uniform behavior with rapid neoplasm, subline L24a, have been reported (22). The autonomous growth, active secretion of TSH, and a sex original L24 pituitary tumor was developed by Dr. Jacob dependence which was lacking in the L24 line. A mam- Furth by I131thyroid ablation. -

SAPNL 2 E.Pdf
2015 2 s newsletter World Pathology Day 3 Sjogren’s syndrome 4 SA antibiogram 6 InSIDe Pathology at your fingertips 8 Donating bone tissue 10 Genetic testing 10 Respiratory virus update 12 For our patients and our population www.sapathology.sa.gov.au From the Respiratory serology changes SA Pathology is decommissioning two antibody test services, the inside Chlamydophila pneumoniae and Respiratory Viruses by Complement This is a time of massive change for the Fixation Tests (CFT). From the Executive Director health system, including for SA Pathology, with the impacts of a burgeoning older Alternatives population and increased demand for You are encouraged to request timely access to affordable and quality PCR tests for faster, more sensitive pathology services, coupled with budget diagnosis of respiratory viruses, pressures on the health system and the M. pneumoniae and B. pertussis. implementation of new computer systems Serological tests for other pathogens and improved pathology automation. including Legionella, M. pneumonia and B. pertussis are not affected. In line with the Transforming Health initiative’s need to provide ‘the best care, Respiratory virus and Chlamydophila first time, every time’, our services will serology is now only available on continuously evolve and improve to focus paired samples and if required, CFT on achieving better outcomes for our testing can be arranged. patient population in partnership with all our Local Health networks and clinicians. Why the change? Whilst antibody testing can provide With the delivery of new e-Health systems a diagnosis of infection a definitive we are pleased to bring you this and analysers designed to handle millions result requires evidence of rising issue of the SA Pathology Newsletter, of tests every year, it is imperative our antibody levels in specimens collected the first for 2015, and trust you will organisation is ready when the new Royal two weeks apart. -

Leukemias Leukemia - Tumor Disease of the Blood System Is Characterized by Three Main Features: 1
Leukemias Leukemia - tumor disease of the blood system is characterized by three main features: 1. Hyperplasia - proliferation of tumor, or a germ hematopoiesis in places where it should be in a normal state (in the bone marrow); 2. Anaplasia - is when the processes of proliferation (cell proliferation) prevails over the differentiation process; 3. Metaplasia - tumor proliferation, of any germ hematopoiesis in places where it should not be (inside the body, the brain, etc.), with an expanding germ hematopoiesis displaces local tissue elements, replacing them with a. Currently, this process is called metastasis. Unlike leucocytosis, leukemoid reactions and other reactive hematopoietic tissue growths at the base of leukemia is uncontrolled (unlimited) cell proliferation in violation of their ability to differentiation and maturation. Loss of ability to mature leukemia cells can take place much more than normal blood cells, the number of cycles of division, and that creates a huge cell production that characterizes leukemia. The etiology of leukemia to date is not certain. On the tumor nature of leukemia indicated by the presence of the general laws that unite leukemias and tumors: a violation of the cell's ability to differentiate; morphological and metabolic anaplasia cells; common etiological factors contributing to the development of leukemia and tumors, and others. The possible etiologic factors causing the development of leukemia, include ionizing radiation, a number of chemicals, viruses. Some importance in the development of leukemia is attached to genetic factors, hereditary and acquired immune deficiency action blastomogenic metabolite of tryptophan and tyrosine. Theories of the origin of leukemia. Radiation theory. The role of ionizing radiation in causing leukemia was proved experimentally.