The University of Adelaide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SA Health Job Pack
SA Health Job Pack Data Entry, Call Centre, Courier - Casual Pool - OPS COVID 19 Job Title - SA Pathology Eligibility Open to Everyone Job Number 763086 Applications Closing Date 31/12/21 Region / Division Statewide Clinical Support Services Health Service SA Pathology Location Metropolitan and Regional positions Classification OPS-1 Job Status Casual opportunities Salary $14.53 - $26.73 per hour plus 25% casual leave loading Contact Details Full name Jill Horn Phone number 7117 2427 Email address [email protected] Criminal History Assessment Applicants will be required to demonstrate that they have undergone an appropriate criminal and relevant history screening assessment/ criminal history check. Depending on the role, this may be a Department of Communities and Social Inclusion (DCSI) Criminal History Check and/or a South Australian Police (SAPOL) National Police Check (NPC). The following checks will be required for this role: Working with Children Screening - DHS Vulnerable Person-Related Employment Screening - NPC Aged Care Sector Employment Screening - NPC General Employment Probity Check - NPC Further information is available on the SA Health careers website at www.sahealth.sa.gov.au/careers - see Career Information, or by referring to the nominated contact person below. For Official Use Only – I1-A1 [Template updated May 2018] Page 1 of 15 Immunisation Risk Category A (direct contact with blood or body substances) This role carries specific immunisation requirements. To be eligible for appointment in this role you will be required to meet the immunisation requirements associated with Category A (direct contact with blood or body substances). Please click here for further information on these requirements. -
CCB Annual Report 2014
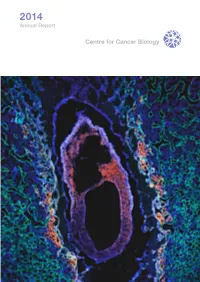
2014 Annual Report Centre for Cancer Biology 2014 Annual Report Centre for Cancer Biology 2 Organisation 3 Alliance Partners Report 4 Centre for Cancer Biology Directors Report 6 Celebrating Our Strategic Alliance Laboratories 8 Acute Leukaemia Laboratory 10 Cell Signalling Laboratory 12 Cytokine Receptor Laboratory 14 Drug Discovery and Development Laboratory 16 Gastroenterology Research Laboratory 18 Gene Regulation Laboratory 20 Hepatitis C Virus Research Laboratory 22 Leukaemia Unit, Genetics and Molecular Pathology 24 Lymphatic Development Laboratory 26 Mast Cell Laboratory 28 Molecular Pathology Research Laboratory 30 Molecular Regulation Laboratory Centre for Cancer Biology 32 Molecular Signalling Laboratory An alliance between 34 Myeloma Research Laboratory SA Pathology and the University of South Australia 36 Neurovascular Research Laboratory SA Pathology 38 Translational Oncology Laboratory Frome Road, Adelaide South Australia 5000 40 Tumour Microenvironment Laboratory Australia 42 Vascular Biology and Cell Trafficking Laboratory T +61 8 8222 3422 F +61 8 8232 4092 44 ACRF Cancer Genomics Facility General Enquiries Ms Anna Nitschke 46 Publications Executive Assistant to Professor Angel Lopez [email protected] 51 Financial Highlights Postal Address 52 New Grants and Fellowships PO Box 14 Rundle Mall Adelaide South Australia 5000 Publication Coordination 54 Seminar Program Australia Anna Nitschke Centre for Cancer Biology 56 Invited Presentations www.centreforcancerbiology.org.au Design and Production Catherine Buddle 58 Awards Buddle Design 60 Research Staff and Students cover image Photography Mark Fitz-Gerald and Peter Dent Sagittal section of an E7.0 embryo surrounded by yolk sac and endometrial tissue SA Pathology Photo & Imaging 62 Our Supporters immunostained to recognise Neuropilin 2 (red), E-cadherin (green) and DAPI (blue). -

Department for Health and Wellbeing 2019-20 Annual Report
OFFICIAL 2019-20 ANNUAL REPORT for the Department for Health and Wellbeing Department for Health and Wellbeing 2019-20 Annual Report Department for Health and Wellbeing PO Box 287 Rundle Mall Adelaide SA 5000 www.sahealth.sa.gov.au Contact phone number: +61 8 8226 0795 Contact email: [email protected] ISSN: 2201-0475 Date presented to Minister: 30 September 2020 1 | P a g e OFFICIAL OFFICIAL 2019-20 ANNUAL REPORT for the Department for Health and Wellbeing To: Hon Stephen Wade MLC Minister for Health and Wellbeing This annual report will be presented to Parliament to meet the statutory reporting requirements of the Public Sector Act 2009, the Public Sector Regulations 2010 and the Public Finance and Audit Act 1987 and the requirements of Premier and Cabinet Circular PC013 Annual Reporting. This report is verified to be accurate for the purposes of annual reporting to the Parliament of South Australia. Submitted on behalf of the Department for Health and Wellbeing by: Dr Christopher McGowan Chief Executive Date______________28-09-2020 Signature ________________ 2 | P a g e OFFICIAL OFFICIAL 2019-20 ANNUAL REPORT for the Department for Health and Wellbeing From the Chief Executive The Department for Health and Wellbeing (the department) as system leader, is committed to supporting the delivery of high quality public health services. Safety, equity, access and responsiveness of services are areas of focus for operations and service delivery to benefit the health and wellbeing of all South Australians. Across the year, the department has continued to improve whole-of-system capability and performance through partnership and connectivity. -

ITA-Biomed Bone and Joint-SA Meeting Adelaide South Australia 21-23 February 2011
Promoting opportunities for scientific and commercial collaboration in biomedical bone and joint research between Italy and South Australia 21-23 February 2011 Adelaide South Australia The University of Adelaide, Union House ITA-Biomed Bone and Joint-SA Meeting Adelaide, South Australia, 21-23 February 2011 The meeting organisers would like to acknowledge the support received from: Financial Support The TM Endorsed by From the chairman of the organising committee Dear delegates, Welcome to all the speakers that have accepted the invitation to attend this meeting. In particular welcome to our guests and speakers from Italy. I hope that all delegates will find their attendance a stimulating research experience and that it presents new research opportunities for collaboration with researchers in South Australia. The aim of this meeting is to promote bilateral scientific and commercial collaboration in biomedical bone and joint research between leading Italian, South Australian and Australian researchers and research organisations. As leaders of research groups covering areas of biomedical research focussing on bone and joint research, you have been invited to explore your combined research capabilities, through this face-to-face networking opportunity, to promote bilateral international collaboration. The meeting has been planned for a selected audience of about 25 participants, playing key roles in their area of research interest. Selected Italian research group leaders and scientists have been invited, along with selected research group leaders and scientists from South Australia and Australia (SA Pathology, Royal Adelaide Hospital, Women’s & Children’s Hospital, The University of Adelaide, Flinders University, University of South Australia, University of Sydney). We also have the participation of industry representatives from Xradia (Pleasanton, CA, USA), who will present to the audience the state of their R&D programs, and their vision or opportunities for collaboration. -

SAPNL 2 E.Pdf
2015 2 s newsletter World Pathology Day 3 Sjogren’s syndrome 4 SA antibiogram 6 InSIDe Pathology at your fingertips 8 Donating bone tissue 10 Genetic testing 10 Respiratory virus update 12 For our patients and our population www.sapathology.sa.gov.au From the Respiratory serology changes SA Pathology is decommissioning two antibody test services, the inside Chlamydophila pneumoniae and Respiratory Viruses by Complement This is a time of massive change for the Fixation Tests (CFT). From the Executive Director health system, including for SA Pathology, with the impacts of a burgeoning older Alternatives population and increased demand for You are encouraged to request timely access to affordable and quality PCR tests for faster, more sensitive pathology services, coupled with budget diagnosis of respiratory viruses, pressures on the health system and the M. pneumoniae and B. pertussis. implementation of new computer systems Serological tests for other pathogens and improved pathology automation. including Legionella, M. pneumonia and B. pertussis are not affected. In line with the Transforming Health initiative’s need to provide ‘the best care, Respiratory virus and Chlamydophila first time, every time’, our services will serology is now only available on continuously evolve and improve to focus paired samples and if required, CFT on achieving better outcomes for our testing can be arranged. patient population in partnership with all our Local Health networks and clinicians. Why the change? Whilst antibody testing can provide With the delivery of new e-Health systems a diagnosis of infection a definitive we are pleased to bring you this and analysers designed to handle millions result requires evidence of rising issue of the SA Pathology Newsletter, of tests every year, it is imperative our antibody levels in specimens collected the first for 2015, and trust you will organisation is ready when the new Royal two weeks apart. -
SA Pathology Newsletter July 2021
SA Pathology Newsletter July 2021 COVID-19 response This week SA Pathology surpassed its highest COVID-19 testing numbers, reporting a record 86,967 tests in the last 7 days; that’s almost double the number of tests reported in the week prior. Our testing numbers peaked at 16,530 on Thursday 22 July – that’s over 10,000 more tests than our recent daily average. At the same time, we also established another COVID-19 drive through collection centre at Waterworld Ridgehaven, an invitation-only priority COVID-19 testing site, and a walk-in collection centre at Netball SA Mile End. SA Pathology also operates COVID-19 collection centres at the Adelaide Airport, Victoria Park, Aldinga GP Plus, Hampstead Rehabilitation Centre and Port Adelaide – refer to the SA Health website for more information – as well as our domiciliary service operating across the metropolitan and regional areas. Working in collaboration with SA Health and Simplybook.me, our Ridgehaven Waterworld and Repat Health Precinct collection centres also began trialling a new testing booking system. The feedback has been extremely positive and we’ve seen a significant reduction in queueing and wait times at these sites. For more information, see the COVID Testing Online Booking FAQs. Mission to fight deadly childhood cancer SA Pathology researchers at the Centre for Cancer Biology (an alliance of SA Pathology and University of South Australia) were recently awarded $1.4 million to investigate the causes and identify new treatments for neuroblastoma in the latest round of Medical Research Future Fund grants. Project lead, Associate Professor Yeesim Khew-Goodall and her co- investigator Professor Greg Goodall (pictured), will work with teams from the University of South Australia, Women’s and Children’s Hospital and Royal Adelaide Hospital as they seek to better understand the causes of neuroblastoma and improve patient outcomes. -

SA Pathology Newsletter (Formerly SA Pathology Will Be Explicitly Moving the IMVS Newsletter)
2013 1 s Newsletter Reference interval changes 2 Significance of ANCA 4 Type 1 diabetes 6 INSIDe Clinical Utility of Bone Turnover Markers 8 Ulysses Syndrome – is it the liver? 10 Test ordering standardisation 11 Order of Draw Quick Guide 12 DNA $ INRTroponineGFR $LDESR CBE HDLPCR $ $ PCRMBA LFT LFTMC&SPSARNA $ HbA1cRCF $ TFT For our Capatients and our population www.sapathology.sa.gov.au From the inside transport improvement, will enable us to Complement method change From the Executive Director meet future challenges and maintain our hard earned reputation. To improve turnaround times for complement C3 and C4 results, As part of a highly regarded health system SA Pathology changed the method that provides outstanding patient care, and analyser platform to the integrated research and teaching activities ADVIA 2400 on 26 August 2013. we aim to continually improve the depth Results for both C3 and C4 using the and breadth of our services whilst returning new method are approximately value for money to South Australian 10% higher than those of the old taxpayers. method. The reference interval has As a clinical support service SA Pathology changed to reflect this shift and a recognises that it needs to positively paediatric range is included. IMVS IS headed by ProfeSSor ruth SaloM a MedIcal graduate respond to the changing clinical landscape who haS SPecIalISed aS a in order to meet the needs of hospitals, C3 SurgIcal PathologISt. clinicians and the community. We also recognise the need to manage demand and <4 weeks 0.58 to 1.08 g/L budget pressures, while ensuring our plans <3 months 0.67 to 1.24 g/L are consistent with the major developments <6 months 0.74 to 1.38 g/L MR KEN BARR IS EXECUTIVE that are occurring within the health system, DIRECTOR OF SA PATHOLOGY including the new Royal Adelaide Hospital <9 months 0.78 to 1.44 g/L and the South Australian Health and <10 years 0.80-1.50 g/L Medical Research Institute (SAHMRI), plus the major research and teaching goals >10 years and 0.85-1.60 g/L of the three universities. -

Final City Qualifying Results
th 39YEAR Popeye Torrens Parade on the torrens Ground FINAL CITY adelaideQUALIFYING RESULTS The be active Corporate Cup is owned by Achper SA Branch and organised by ‘Life. Be in it’ SA Final City Qualifying Results Thank you for being part of the 2019 Corporate Cup! We’d like to thank everyone who participated in the Corporate Cup this year. Whether you achieved your fitness goals or are still trying we hope you found the event encouraging. As the true spirit of the event is getting out there with others and having a go! And of course, congratulations to all the category winners for 2019. A big thank you must also go to all of our event partners and sponsors; Finally, a huge thanks to ACHPER SA (owners of the Corporate Cup) for continuing to trust management of the event to Enventive/Life. Be in it™. We look forward to seeing you all again next year! :) – from the ‘Life. Be in it’ team Final City Qualifying Results be active Corporate Cup Honour Roll Life members Additional 20 year club members cont. Peter Lomman Aurecon John George Mutual Community, Work Cover, Elders Euan Mackenzie Individual (Ex ABC) Tricia Anderson TAFE SA John Merrit ABC Peter Clark ATO Janet Leitch Don Penno Fredericka Giamarelos 30 Consecutive years honour roll Michael Bertelmeier Telecom / Health SA David Bridges Retired from Parliament House Grace Finlay Individual Rennie Hamilton SGIC now QBE David Ball Dept Families and Communities Peter Lomman Connell Wagner now Aurecon Engineering Ruth Byrnes Transport SA Euan MacKenzie ABC now individual runner Winton Foale -

SA Pathology Newsletter 3 Issue 3
2015 3 s Newsletter Maternal GBS detection 3 New genetics technology 4 SA Pathology INSIde in the new RAH 5 Haemoglobinopathy 6 Vitamin B12 deficiency 8 Aspergillosis infection 10 Respiratory virus update 12 Wallaroo Mt Gambier > 54 Port Augusta > 59 YeARS > 45 YeARS YeARS Berri > 46 We’re part of YeARS your community Gawler Port Pirie > 35 Murray Bridge YeARS > 29 YeARS > 41 YeARS Victor Harbor Whyalla > 27 Port Lincoln > 50 YeARS > 47 YeARS YeARS For our patients and our population www.sapathology.sa.gov.au TFT or TSH From the When considering thyroid testing consider whether you should request inside a TFT or a TSH? A request for TFT allows us to robotics technology, which will all provide you with a fT4 test where From the Executive Director work together with the new patient the TSH is abnormal or the tests are administration system (ePAS) being performed: introduced across our SA hospitals. n for the purpose of monitoring The convergence and integration of thyroid disease in the patient; or technologies puts us under pressure n to investigate the sick euthyroid to achieve time lines and agreed syndrome if the patient is an service benchmarks, but also presents admitted patient; or opportunities to embrace change for n to investigate dementia or better patient outcomes. psychiatric illness of the patient; We now have a chance to improve on or the best diagnostic support for all South n to investigate amenorrhoea or Australians, ensuring we are there at the infertility of the patient; or right time for the right clinical reason, n you suspect your patient has a and to support healthcare professionals pituitary dysfunction or making their decisions and tasks easier, n your patient is on drugs that resulting in improved care for our interfere with thyroid hormone few businesses today are patients and the population. -

CML Handbook of Chronic Myeloid Leukemia
Hughes · Ross Melo Timothy P Hughes · David M Ross · Junia V Melo Handbook of Chronic Myeloid Leukemia Myeloid Handbook of Chronic Handbook of Chronic Myeloid Leukemia Timothy P Hughes · David M Ross · Junia V Melo Handbook of Chronic Myeloid Leukemia This independent educational resource is supported by a grant from Novartis Oncology. Timothy P Hughes · David M Ross · Junia V Melo Handbook of Chronic Myeloid Leukemia Timothy P Hughes MD, FRACP, FRCPA, MBBS David M Ross MBBS, PhD, FRACP, FRCPA SA Pathology & SAHMRI University of Adelaide and Flinders University University of Adelaide SA Pathology Department of Hematology Department of Hematology Adelaide Adelaide Australia Australia Junia V Melo MD, PhD, FRCPath University of Adelaide Department of Hematology Adelaide Australia ISBN 978-3-319-08349-0 ISBN 978-3-319-08350-6 (eBook) DOI 10.1007/978-3-319-08350-6 © Springer International Publishing Switzerland 2016 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. -

Lincoln Line Issue 53
LincolnLine issue 53 | spring 2013 in memory of Dr Geoffrey Scott... page 4 40 years of women at college alumni dinner 2013 arts at lincoln contents events from the chairman ............................................ 3 22 February 2014: Move In Day - Welcome in memory of Dr Scott ...................................... 4 Freshers! arts at lincoln ..................................................... 6 thank you for your support .............................. 9 23 February 2014: Move In Day - Returning 40 years of women .......................................... 10 Residents scholarships .................................................... 12 24 February - 2 March 2014 : O-Week academic ......................................................... 14 creative writing .............................................. 16 5 May 2014: Scholarship Dinner boys’ night in & girls’ night in ...................... 18 31 May 2014: Annual Alumni Dinner - SAVE meet the council ............................................. 19 THE DATE! LCAA & you .................................................... 20 alumni dinner 2013 ......................................... 22 lincoln alumni wine club ................................ 24 alumni story .................................................... 25 Find us on Facebook thanks for dropping in .................................... 27 Lincoln College, alumni news .................................................... 28 North Adelaide Lincoln Line Lincoln Line is the official magazine of Lincoln College. It provides news -
CCB Annual Report 2013
2013 Annual Report Centre for Cancer Biology 2013 Annual Report Centre for Cancer Biology 2 Organisation 3 SA Pathology Executive Director’s Report 4 Centre for Cancer Biology Directors’ Report 6 6th Barossa Meeting Laboratories 8 Acute Leukaemia Laboratory 10 Cell Signalling Laboratory 12 Cytokine Receptor Laboratory 14 Gastroenterology Research Laboratory 16 Gene Regulation Laboratory 18 Haematology Clinical Research Unit 20 Hepatitis C Virus Research Laboratory 22 Leukaemia Unit, Genetics and Molecular Pathology 24 Lymphatic Development Laboratory 26 Mast Cell Laboratory 28 Melissa White Memorial Laboratory 30 Molecular Pathology Research Laboratory 32 Molecular Regulation Laboratory Centre for Cancer Biology 34 Molecular Signalling Laboratory SA Pathology Frome Road, Adelaide 36 Myeloma Research Laboratory South Australia 5000 Australia 38 Neurovascular Research Laboratory T +61 8 8222 3422 40 Translational Oncology Laboratory F +61 8 8232 4092 42 Tumour Microenvironment Laboratory General Enquiries Ms Anna Nitschke 44 Vascular Biology and Cell Trafficking Laboratory Executive Assistant to Professor Angel Lopez [email protected] 46 ACRF Cancer Genomics Facility Postal Address PO Box 14 Rundle Mall 48 Publications Adelaide South Australia 5000 Australia 53 Financial Highlights www.centreforcancerbiology.org.au 54 New Grants and Fellowships 56 Seminar Program 58 Invited Presentations Publication Coordination Anna Nitschke Centre for Cancer Biology 61 Awards Design and Production 62 Research Staff and Students Catherine Buddle