Once-Daily Single-Inhaler Versus Twice-Daily Multiple-Inhaler Triple Therapy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Advantages and Disadvantages of Beta- Adrenergic Blocking Drugs in Hypertension
Reprinted from ANCIOLOCY Vol. 29, No. -I April 1978 Copyright 0 1978 Prinred in U.S.A. All Rights Rewrced Advantages and Disadvantages of Beta- Adrenergic Blocking Drugs in Hypertension Eoin T. O'Brien DUBLIN, IRELAND General Measures Elevation of blood pressure should be regarded as one of a number of potential risk factors for cardiovascular disease-albeit a major risk factor- rather than a disease per se.' It is important to identify additional risk factors in the hypertensive patient, not only because collectively these factors may greatly magnify the cardiovascular risk, but also because modification of them may, of itself, lower the blood pressure and thus alleviate the risk and save the patient the inconvenience, expense, and potential harm that may result from even the simplest of drug regimes. Careful consideration should be given to the patient's diet (particularly in relation to the calorie intake in the case of obesity, the cholesterol and saturated fat content in the case of hyperlipidemia and patients at high risk, and the salt content) and to smoking habits, physical activity. stress. personality, and drug therapy, especially anovulant preparations. Other diseases, such as diabetes mellitus, which are associated with a high incidence of hypertension and pri- mary causes of hypertension must be excluded. Although there is still no statistical evidence to show that modification of these risk factors-with the exception of tobacco and anovulant preparations-will actually reduce mortal- ity, it does seem prudent on the basis of the evidence available to encourage the hypertensive patient to adjust his or her life-style not only to reduce the cardiovascular risk,2 but also because in many instances the mildly hypertensive patient will respond to this approach alone. -

Drug Class Review Antianginal Agents
Drug Class Review Antianginal Agents 24:12.08 Nitrates and Nitrites 24:04.92 Cardiac Drugs, Miscellaneous Amyl Nitrite Isosorbide Dinitrate (IsoDitrate ER®, others) Isosorbide Mononitrate (Imdur®) Nitroglycerin (Minitran®, Nitrostat®, others) Ranolazine (Ranexa®) Final Report May 2015 Review prepared by: Melissa Archer, PharmD, Clinical Pharmacist Carin Steinvoort, PharmD, Clinical Pharmacist Gary Oderda, PharmD, MPH, Professor University of Utah College of Pharmacy Copyright © 2015 by University of Utah College of Pharmacy Salt Lake City, Utah. All rights reserved. Table of Contents Executive Summary ......................................................................................................................... 3 Introduction .................................................................................................................................... 4 Table 1. Antianginal Therapies .............................................................................................. 4 Table 2. Summary of Agents .................................................................................................. 5 Disease Overview ........................................................................................................................ 8 Table 3. Summary of Current Clinical Practice Guidelines .................................................... 9 Pharmacology ............................................................................................................................... 10 Table 4. Pharmacokinetic Properties -

COPD Agents Review – October 2020 Page 2 | Proprietary Information
COPD Agents Therapeutic Class Review (TCR) October 1, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. October 2020 -

CORGARD® TABLETS Nadolol Tablets USP
CORGARD® TABLETS Nadolol Tablets USP Rx Only DESCRIPTION CORGARD (nadolol) is a synthetic nonselective beta-adrenergic receptor blocking agent designated chemically as 1-(tert-butylamino)-3-[(5,6,7,8-tetrahydro-cis-6,7-dihydroxy-1- naphthyl)oxy]-2-propanol. Structural formula: C17H27NO4 MW 309.40 Nadolol is a white crystalline powder. It is freely soluble in ethanol, soluble in hydrochloric acid, slightly soluble in water and in chloroform, and very slightly soluble in sodium hydroxide. CORGARD (nadolol) is available for oral administration as 20 mg, 40 mg, and 80 mg tablets. Inactive ingredients: microcrystalline cellulose, colorant (FD&C Blue No. 2), corn starch, magnesium stearate, povidone (except 20 mg and 40 mg), and other ingredients. CLINICAL PHARMACOLOGY CORGARD (nadolol) is a nonselective beta-adrenergic receptor blocking agent. Clinical pharmacology studies have demonstrated beta-blocking activity by showing (1) reduction in heart rate and cardiac output at rest and on exercise, (2) reduction of systolic and diastolic blood pressure at rest and on exercise, (3) inhibition of isoproterenol-induced tachycardia, and (4) reduction of reflex orthostatic tachycardia. CORGARD (nadolol) specifically competes with beta-adrenergic receptor agonists for available beta receptor sites; it inhibits both the beta1 receptors located chiefly in cardiac muscle and the beta2 receptors located chiefly in the bronchial and vascular musculature, inhibiting the chronotropic, inotropic, and vasodilator responses to beta-adrenergic stimulation proportionately. CORGARD has no intrinsic sympathomimetic activity and, unlike some other beta-adrenergic blocking agents, nadolol has little direct myocardial depressant activity and does not have an anesthetic-like membrane- stabilizing action. Animal and human studies show that CORGARD slows the sinus rate and depresses AV conduction. -

Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanismss
Supplemental Material can be found at: /content/suppl/2020/12/18/73.1.202.DC1.html 1521-0081/73/1/202–277$35.00 https://doi.org/10.1124/pharmrev.120.000056 PHARMACOLOGICAL REVIEWS Pharmacol Rev 73:202–277, January 2021 Copyright © 2020 by The Author(s) This is an open access article distributed under the CC BY-NC Attribution 4.0 International license. ASSOCIATE EDITOR: MICHAEL NADER Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanismss Antonio Inserra, Danilo De Gregorio, and Gabriella Gobbi Neurobiological Psychiatry Unit, Department of Psychiatry, McGill University, Montreal, Quebec, Canada Abstract ...................................................................................205 Significance Statement. ..................................................................205 I. Introduction . ..............................................................................205 A. Review Outline ........................................................................205 B. Psychiatric Disorders and the Need for Novel Pharmacotherapies .......................206 C. Psychedelic Compounds as Novel Therapeutics in Psychiatry: Overview and Comparison with Current Available Treatments . .....................................206 D. Classical or Serotonergic Psychedelics versus Nonclassical Psychedelics: Definition ......208 Downloaded from E. Dissociative Anesthetics................................................................209 F. Empathogens-Entactogens . ............................................................209 -

0Bcore Safety Profile
Core Safety Profile Active substance: Levobunolol Pharmaceutical form(s)/strength: Eye drops solution/ 0,1%; 0,25%; 0,5%; 0,5% UD P-RMS: CZ/H/PSUR/0006/001 Date of FAR: 26.05.2009 4.2 Posology and method of administration Adults (including the elderly) Country specific posology and method of administration to be included. Children /.../ is not recommended for use in children due to lack of safety and efficacy data. If required, /.../ may be used with other agents to lower intra-ocular pressure. The use of two topical beta-adrenergic blocking agents is not recommended (see section 4.4). Intraocular pressure should be measured approximately four weeks after starting treatment with /.../ as a return to normal ocular pressure can take a few weeks. As with any eye drops, to reduce possible systemic absorption, it is recommended that the lachrymal sac is compressed at the medial canthus (punctual occlusion) for one minute. This should be performed immediately following the instillation of each drop. Transfer from other beta-blocking treatment When another beta blocking agent is being used treatment must be discontinued after a full day of therapy. Start treatment with /.../ the next day with X drop of /.../ topically applied into the conjunctival sac in the affected eye(s). If /.../ is to replace a combination of anti-glaucoma products, only a single product should be removed at a time. Use in renal and hepatic impairment Levobunolol hydrochloride has not been studied in patients with hepatic or renal impairment. Therefore, caution should be used in treating such patients (see section 4.4). -

Metered-Dose Inhalers (Mdis): Anti-Cholinergic Drugs
Texas Vendor Drug Program Drug Use Criteria: Aerosolized Agents - Metered-Dose Inhalers (MDIs): Anti-Cholinergic Drugs Publication History 1. Developed January 1995. 2. Revised April 2021; March 2019; March 2017; November 2015; March 2014; August 2012; June 2012; October 2010; January 2008; January 2003; January 2002; January 2001; March 2000; January 2000; February 1999; February 1998; February 1997; August 1995. Notes: All criteria may be applied retrospectively. The information contained is for the convenience of the public. The Texas Health and Human Services Commission is not responsible for any errors in transmission or any errors or omissions in the document. Medications listed in the tables and non-FDA approved indications included in these retrospective criteria are not indicative of Vendor Drug Program formulary coverage. Prepared by: • Drug Information Service, UT Health San Antonio. • The College of Pharmacy, The University of Texas at Austin 1 1 Dosage 1.1 Adults Ipratropium (Atrovent®), a short-acting, inhalational anticholinergic agent, is FDA- approved to manage bronchospasm associated with chronic bronchitis and emphysema, collectively known as chronic obstructive pulmonary disease (COPD). Ipratropium is considered a second-line agent in the treatment of asthma as the bronchodilatory effects seen with ipratropium are less than those seen with beta- adrenergic drugs. While not FDA approved, the Expert Panel 3 guidelines from the National Heart Lung and Blood Institute document benefit when multiple ipratropium doses are administered adjunctively with beta2-agonists in the emergency department to manage more severe acute asthma exacerbations, and the Global Initiative for Asthma (GINA) guidelines state that ipratropium may be considered an alternative bronchodilator in patients who experience adverse effects to short-acting beta2-agonists (e.g., tachycardia, arrhythmia, tremor). -
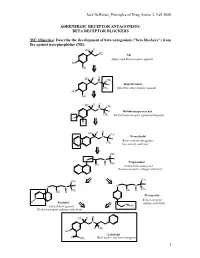
BETA RECEPTOR BLOCKERS MC Objective
Jack DeRuiter, Principles of Drug Action 2, Fall 2000 ADRENERGIC RECEPTOR ANTAGONISTS: BETA RECEPTOR BLOCKERS MC Objective: Describe the development of beta antagonists ("beta blockers") from the agonist norepinephrine (NE): HO H NH2 NE Alpha- and Beta-receptor agonist HO OH H H HO CH N 3 H Isoproterenol CH3 Selective beta-receptor agonist HO OH H H HO CH N 3 H Dichloroisoproterenol CH3 Partial beta-receptor agonist/antagonist Cl Cl H H HO CH N 3 Pronethalol H Beta-receptor antagonist, CH 3 low activity and toxic CH3 O N H Propranolol CH3 OH H Potent beta-antagonist No beta-receptor subtype selectivity CH3 CH3 O N H O N H CH CH OH H 3 OH H 3 Metoprolol N Beta-1-receptor H Pindolol O subtype selectivity Partial beta-agonist CH3 No beta-receptor subtype selectivity HO H H N H CH3 HO Labetolol O NH2 Dual alpha- and beta-antagonst 1 Jack DeRuiter, Principles of Drug Action 2, Fall 2000 MC Objective: Based on their structures, would the beta-blockers be expected to be relatively receptor selective? YES. They do not produce significant blockade of alpha- adrenergic receptors (alpha-1 or alpha-2), histamine receptors, muscarinic receptors or dopamine receptors. MC/PC Objective: Identify which beta blockers are classified as "non-selective": · The “non-selective" classification refers to those beta-blockers capable of blocking BOTH beta-1 and beta-2 receptors with equivalent efficacy. These drugs DO NOT have clinically significant affinity for other neurotransmitter receptors (alpha, dopamine, histamine, acetylcholine, etc.). · ALL of these beta-blockers (except satolol) consist of an aryloxypropanolamine side chain linked to an aromatic or “heteroaromatic” ring which is “ortho” substituted. -

Therapeutic Drug Class
BUREAU FOR MEDICAL SERVICES EFFECTIVE WEST VIRGINIA MEDICAID PREFERRED DRUG LIST WITH PRIOR AUTHORIZATION CRITERIA 07/01/2018 This is not an all-inclusive list of available covered drugs and includes only Version 2018.3e managed categories. Refer to cover page for complete list of rules governing this PDL. • Prior authorization for a non-preferred agent in any class will be given only if there has been a trial of the preferred brand/generic equivalent or preferred formulation of the active ingredient, at a therapeutic dose, that resulted in a partial response with a documented intolerance. • Prior authorization of a non-preferred isomer, pro-drug, or metabolite will be considered with a trial of a preferred parent drug of the same chemical entity, at a therapeutic dose, that resulted in a partial response with documented intolerance or a previous trial and therapy failure, at a therapeutic dose, with a preferred drug of a different chemical entity indicated to treat the submitted diagnosis. (The required trial may be overridden when documented evidence is provided that the use of these preferred agent(s) would be medically contraindicated.) • Unless otherwise specified, the listing of a particular brand or generic name includes all legend forms of that drug. OTC drugs are not covered unless specified. • PA criteria for non-preferred agents apply in addition to general Drug Utilization Review policy that is in effect for the entire pharmacy program, including, but not limited to, appropriate dosing, duplication of therapy, etc. • The use of pharmaceutical samples will not be considered when evaluating the members’ medical condition or prior prescription history for drugs that require prior authorization. -

Albert Hofmann's Pioneering Work on Ergot Alkaloids and Its Impact On
BIRTHDAY 83 CHIMIA 2006, 60, No. 1/2 Chimia 60 (2006) 83–87 © Schweizerische Chemische Gesellschaft ISSN 0009–4293 Albert Hofmann’s Pioneering Work on Ergot Alkaloids and Its Impact on the Search of Novel Drugs at Sandoz, a Predecessor Company of Novartis Dedicated to Dr. Albert Hofmann on the occasion of his 100th birthday Rudolf K.A. Giger* and Günter Engela Abstract: The scientific research on ergot alkaloids is fundamentally related to the work of Dr. Albert Hofmann, who was able to produce, from 1935 onwards, a number of novel and valuable drugs, some of which are still in use today. The complex chemical structures of ergot peptide alkaloids and their pluripotent pharmacological activity were a great challenge for Dr. Hofmann and his associates who sought to unravel the secrets of the ergot peptide alkaloids; a source of inspiration for the design of novel, selective and valuable medicines. Keywords: Aminocyclole · Bromocriptine Parlodel® · Dihydroergotamine Dihydergot® · Dihydro ergot peptide alkaloids · Ergobasin/ergometrin · Ergocornine · Ergocristine · α- and β-Ergocryptine · Ergolene · Ergoline · Ergoloid mesylate Hydergine® · Ergotamine Gynergen® · Ergotoxine · Lisuride · Lysergic acid diethylamide LSD · Methylergometrine Methergine® · Methysergide Deseril® · Paspalic acid · Pindolol Visken® · Psilocybin · Serotonin · Tegaserod Zelmac®/Zelnorm® · Tropisetron Navoban® Fig. 1. Albert Hofmann in 1943, 1979 and 2001 (Photos in 2001 taken by J. Zadrobilek and P. Schmetz) Dr. Albert Hofmann (Fig. 1), born on From the ‘Ergot Poison’ to *Correspondence: Dr. R.K.A. Giger January 11, 1906, started his extremely Ergotamine Novartis Pharma AG NIBR Global Discovery Chemistry successful career in 1929 at Sandoz Phar- Lead Synthesis & Chemogenetics ma in the chemical department directed The scientific research on ergot alka- WSJ-507.5.51 by Prof. -

Medication: Propranolol (Inderal) 10 Mg for POTS (Postural Orthostatic Tachycardia Syndrome)
Propranolol for POTS COMPLEX CHRONIC DISEASES PROGRAM Medication Handout Date: May 15, 2018 Medication: Propranolol (Inderal) 10 mg for POTS (Postural Orthostatic Tachycardia Syndrome) What is propranolol: Propranolol is a type of medication known as a “beta-blocker:” blocks adrenaline and related chemicals which can be used for Postural Orthostatic Tachycardia Syndrome (POTS) by reducing the heart rate. It is also helpful for migraine prevention but sometimes the dose used for POTS is not enough for migraines, and higher doses may make POTS patients dizzier. Propranolol is also helpful with the physical effects of anxiety. Expected benefit: Usually takes a day or two to notice a benefit once you are on the optimal dose Watch for possible side effects: This list of side effects is important for you to be aware of however, it is also important to remember that not all side effects happen to everyone. If you have problems with these side effects talk with your doctor or pharmacist: Dizziness Low heart rate Low blood pressure Fatigue Low mood Stopping the medication: This medication should not be stopped abruptly if being used regularly or at higher doses. Your doctor may advise you to reduce the dose slowly. How to use this medication: Take this medication with or without food Dosing Schedule: Start with 10 mg as a “test” dose to ensure you do not experience dizziness Then start 10 mg 3 – 4 times a day In some cases, it can be used as needed rather than regularly You can increase the dose to 20 mg 3 – 4 times a day if needed Drugs and Foods to Avoid: Ask your doctor or pharmacist before using any other medication, including non- prescription medication (over-the-counter medication) and herbal products. -

Beta-Blockers for Hypertension: Time to Call a Halt
Journal of Human Hypertension (1998) 12, 807–810 1998 Stockton Press. All rights reserved 0950-9240/98 $12.00 http://www.stockton-press.co.uk/jhh FOR DEBATE Beta-blockers for hypertension: time to call a halt DG Beevers University Department of Medicine, City Hospital, Birmingham B18 7QH, UK Beta-blockers are not very effective at lowering blood the endorsement of beta-blockers by the British Hyper- pressure in elderly hypertensive patients or in Afro- tension Society and other guidelines committees, Caribbeans and these two groups represent a large pro- except possibly for severe resistant hypertension, high portion of people with raised blood pressure. Further- risk post-infarct patients and those with angina pectoris. more they do not prevent more heart attacks than the The time has come to move across to newer, safer, more thiazide diuretics. Beta-blockers can also be dangerous tolerable and more effective antihypertensive agents in many hypertensive patients and even when these whilst continuing to use thiazide diuretics in low doses drugs are not contraindicated, they cause subtle and in the elderly as first choice, providing there are no depressing side effects which should preclude their contraindications. usefulness. The time has come therefore to reconsider Keywords: beta-blockers; hypertension Introduction Safety and tolerability Beta-adrenergic blockers were first introduced in the There is little doubt that the beta-blockers are the early 1960s for the treatment of angina pectoris. most unsafe of all antihypertensive drugs. They can Their antihypertensive properties were not fully precipitate or worsen heart failure in patients with recognised until the celebrated paper by Pritchard myocardial damage and they are contraindicated in and Gillam in 1964.1 They rapidly became popular patients with asthma.