0Bcore Safety Profile
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intraocular Pressure Rise After Phacoemulsification Prophylactic
British Journal of Ophthalmology 1995; 79: 809-813 809 Intraocular pressure rise after phacoemulsification Br J Ophthalmol: first published as 10.1136/bjo.79.9.809 on 1 September 1995. Downloaded from with posterior chamber lens implantation: effect of prophylactic medication, wound closure, and surgeon's experience Thomas G Bomer, Wolf-Dietrich A Lagreze, Jens Funk Abstract pressure rises usually occur between 6 and 8 Aims-A prospective clinical trial was hours after surgery.6 carried out to evaluate the effect of Various antiglaucomatous agents have been prophylactic medication, the technique of used to prevent the intraocular pressure rise wound closure, and the surgeon's experi- after cataract extraction. Oral acetazolamide15 16 ence on the intraocular pressure rise after and topical timolol'6-19 lowered the pressure cataract extraction. rise in the early period after intracapsular and Methods-In 100 eyes, the intraocular extracapsular cataract extraction. Levobunolol pressure was measured before as well as proved to be superior to timolol 4-7 hours 2-4, 5-7, and 22-24 hours after phaco- after extracapsular cataract extraction.20 Apra- emulsification and posterior chamber lens clonidine lowered the intraocular pressure rise implantation. Each of 25 patients received after uncomplicated phacoemulsification21 and either 1% topical apraclonidine, 0.5%/o extracapsular cataract extraction22 23 when topical levobunolol, 500 mg oral acetazo- given 30 minutes to 1 hour before surgery, lamide, or placebo. Forty four eyes were whereas immediate postoperative treatment operated with sclerocorneal sutureless with apraclonidine was ineffective.22 24 Miotics tunnel and 56 eyes with corneoscleral are frequently used to promote miosis and incision and suture. -

Brimonidine Tartrate; Brinzolamide
Contains Nonbinding Recommendations Draft Guidance on Brimonidine Tartrate ; Brinzolamide This draft guidance, when finalized, will represent the current thinking of the Food and Drug Administration (FDA, or the Agency) on this topic. It does not establish any rights for any person and is not binding on FDA or the public. You can use an alternative approach if it satisfies the requirements of the applicable statutes and regulations. To discuss an alternative approach, contact the Office of Generic Drugs. Active Ingredient: Brimonidine tartrate; Brinzolamide Dosage Form; Route: Suspension/drops; ophthalmic Strength: 0.2%; 1% Recommended Studies: One study Type of study: Bioequivalence (BE) study with clinical endpoint Design: Randomized (1:1), double-masked, parallel, two-arm, in vivo Strength: 0.2%; 1% Subjects: Males and females with chronic open angle glaucoma or ocular hypertension in both eyes. Additional comments: Specific recommendations are provided below. ______________________________________________________________________________ Analytes to measure (in appropriate biological fluid): Not applicable Bioequivalence based on (95% CI): Clinical endpoint Additional comments regarding the BE study with clinical endpoint: 1. The Office of Generic Drugs (OGD) recommends conducting a BE study with a clinical endpoint in the treatment of open angle glaucoma and ocular hypertension comparing the test product to the reference listed drug (RLD), each applied as one drop in both eyes three times daily at approximately 8:00 a.m., 4:00 p.m., and 10:00 p.m. for 42 days (6 weeks). 2. Inclusion criteria (the sponsor may add additional criteria): a. Male or nonpregnant females aged at least 18 years with chronic open angle glaucoma or ocular hypertension in both eyes b. -

Advantages and Disadvantages of Beta- Adrenergic Blocking Drugs in Hypertension
Reprinted from ANCIOLOCY Vol. 29, No. -I April 1978 Copyright 0 1978 Prinred in U.S.A. All Rights Rewrced Advantages and Disadvantages of Beta- Adrenergic Blocking Drugs in Hypertension Eoin T. O'Brien DUBLIN, IRELAND General Measures Elevation of blood pressure should be regarded as one of a number of potential risk factors for cardiovascular disease-albeit a major risk factor- rather than a disease per se.' It is important to identify additional risk factors in the hypertensive patient, not only because collectively these factors may greatly magnify the cardiovascular risk, but also because modification of them may, of itself, lower the blood pressure and thus alleviate the risk and save the patient the inconvenience, expense, and potential harm that may result from even the simplest of drug regimes. Careful consideration should be given to the patient's diet (particularly in relation to the calorie intake in the case of obesity, the cholesterol and saturated fat content in the case of hyperlipidemia and patients at high risk, and the salt content) and to smoking habits, physical activity. stress. personality, and drug therapy, especially anovulant preparations. Other diseases, such as diabetes mellitus, which are associated with a high incidence of hypertension and pri- mary causes of hypertension must be excluded. Although there is still no statistical evidence to show that modification of these risk factors-with the exception of tobacco and anovulant preparations-will actually reduce mortal- ity, it does seem prudent on the basis of the evidence available to encourage the hypertensive patient to adjust his or her life-style not only to reduce the cardiovascular risk,2 but also because in many instances the mildly hypertensive patient will respond to this approach alone. -

Drug Class Review Antianginal Agents
Drug Class Review Antianginal Agents 24:12.08 Nitrates and Nitrites 24:04.92 Cardiac Drugs, Miscellaneous Amyl Nitrite Isosorbide Dinitrate (IsoDitrate ER®, others) Isosorbide Mononitrate (Imdur®) Nitroglycerin (Minitran®, Nitrostat®, others) Ranolazine (Ranexa®) Final Report May 2015 Review prepared by: Melissa Archer, PharmD, Clinical Pharmacist Carin Steinvoort, PharmD, Clinical Pharmacist Gary Oderda, PharmD, MPH, Professor University of Utah College of Pharmacy Copyright © 2015 by University of Utah College of Pharmacy Salt Lake City, Utah. All rights reserved. Table of Contents Executive Summary ......................................................................................................................... 3 Introduction .................................................................................................................................... 4 Table 1. Antianginal Therapies .............................................................................................. 4 Table 2. Summary of Agents .................................................................................................. 5 Disease Overview ........................................................................................................................ 8 Table 3. Summary of Current Clinical Practice Guidelines .................................................... 9 Pharmacology ............................................................................................................................... 10 Table 4. Pharmacokinetic Properties -

Table 1. Glaucoma Medications: Mechanisms, Dosing and Precautions Brand Generic Mechanism of Action Dosage/Avg
OPTOMETRIC STUDY CENTER Table 1. Glaucoma Medications: Mechanisms, Dosing and Precautions Brand Generic Mechanism of Action Dosage/Avg. % Product Sizes Side Effects Warnings Reduction CHOLINERGIC AGENTS Direct Pilocarpine (generic) Pilocarpine 1%, 2%, 4% Increases trabecular outflow BID-QID/15-25% 15ml Headache, blurred vision, myopia, retinal detachment, bronchiole constriction, Angle closure, shortness of breath, retinal narrowing of angle detachment Indirect Phospholine Iodide (Pfizer) Echothiophate iodide 0.125% Increases trabecular outflow QD-BID/15-25% 5ml Same as above plus cataractogenic iris cysts in children, pupillary block, Same as above, plus avoid prior to any increased paralysis with succinylcholine general anesthetic procedure ALPHA-2 AGONISTS Alphagan P (Allergan) Brimonidine tartrate 0.1%, 0.15% with Purite Decreases aqueous production, increases BID-TID/up to 26% 5ml, 10ml, 15ml Dry mouth, hypotension, bradycardia, follicular conjunctivitis, ocular irritation, Monitor for shortness of breath, dizziness, preservative uveoscleral outflow pruritus, dermatitis, conjunctival blanching, eyelid retraction, mydriasis, drug ocular redness and itching, fatigue allergy Brimonidine tartrate Brimonidine tartrate 0.15%, 0.2% Same as above Same as above 5ml, 10ml Same as above Same as above (generic) Iopidine (Novartis) Apraclonidine 0.5% Decreases aqueous production BID-TID/up to 25% 5ml, 10ml Same as above but higher drug allergy (40%) Same as above BETA-BLOCKERS Non-selective Betagan (Allergan) Levobunolol 0.25%, 0.5% Decreases -

COPD Agents Review – October 2020 Page 2 | Proprietary Information
COPD Agents Therapeutic Class Review (TCR) October 1, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. October 2020 -

NEW ZEALAND DATA SHEET 1. PRODUCT NAME IOPIDINE® (Apraclonidine Hydrochloride) Eye Drops 0.5%
NEW ZEALAND DATA SHEET 1. PRODUCT NAME IOPIDINE® (apraclonidine hydrochloride) Eye Drops 0.5%. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each mL of Iopidine Eye Drops 0.5% contains apraclonidine hydrochloride 5.75 mg, equivalent to apraclonidine base 5 mg. Excipient with known effect Benzalkonium chloride 0.1 mg per 1 mL as a preservative. For the full list of excipients, see section 6.1. 2. PHARMACEUTICAL FORM Eye drops, solution, sterile, isotonic. 4. CLINICAL PARTICULARS 4.1. Therapeutic indications Iopidine Eye Drops 0.5% are indicated for short-term adjunctive therapy in patients on maximally tolerated medical therapy who require additional IOP reduction. Patients on maximally tolerated medical therapy who are treated with Iopidine Eye Drops 0.5% to delay surgery should have frequent follow up examinations and treatment should be discontinued if the intraocular pressure rises significantly. The addition of Iopidine Eye Drops 0.5% to patients already using two aqueous suppressing drugs (i.e. beta-blocker plus carbonic anhydrase inhibitor) as part of their maximally tolerated medical therapy may not provide additional benefit. This is because apraclonidine is an aqueous-suppressing drug and the addition of a third aqueous suppressant may not significantly reduce IOP. The IOP-lowering efficacy of Iopidine Eye Drops 0.5% diminishes over time in some patients. This loss of effect, or tachyphylaxis, appears to be an individual occurrence with a variable time of onset and should be closely monitored. The benefit for most patients is less than one month. 4.2. Dose and method of administration Dose One drop of Iopidine Eye Drops 0.5% should be instilled into the affected eye(s) three times per day. -

CORGARD® TABLETS Nadolol Tablets USP
CORGARD® TABLETS Nadolol Tablets USP Rx Only DESCRIPTION CORGARD (nadolol) is a synthetic nonselective beta-adrenergic receptor blocking agent designated chemically as 1-(tert-butylamino)-3-[(5,6,7,8-tetrahydro-cis-6,7-dihydroxy-1- naphthyl)oxy]-2-propanol. Structural formula: C17H27NO4 MW 309.40 Nadolol is a white crystalline powder. It is freely soluble in ethanol, soluble in hydrochloric acid, slightly soluble in water and in chloroform, and very slightly soluble in sodium hydroxide. CORGARD (nadolol) is available for oral administration as 20 mg, 40 mg, and 80 mg tablets. Inactive ingredients: microcrystalline cellulose, colorant (FD&C Blue No. 2), corn starch, magnesium stearate, povidone (except 20 mg and 40 mg), and other ingredients. CLINICAL PHARMACOLOGY CORGARD (nadolol) is a nonselective beta-adrenergic receptor blocking agent. Clinical pharmacology studies have demonstrated beta-blocking activity by showing (1) reduction in heart rate and cardiac output at rest and on exercise, (2) reduction of systolic and diastolic blood pressure at rest and on exercise, (3) inhibition of isoproterenol-induced tachycardia, and (4) reduction of reflex orthostatic tachycardia. CORGARD (nadolol) specifically competes with beta-adrenergic receptor agonists for available beta receptor sites; it inhibits both the beta1 receptors located chiefly in cardiac muscle and the beta2 receptors located chiefly in the bronchial and vascular musculature, inhibiting the chronotropic, inotropic, and vasodilator responses to beta-adrenergic stimulation proportionately. CORGARD has no intrinsic sympathomimetic activity and, unlike some other beta-adrenergic blocking agents, nadolol has little direct myocardial depressant activity and does not have an anesthetic-like membrane- stabilizing action. Animal and human studies show that CORGARD slows the sinus rate and depresses AV conduction. -

Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanismss
Supplemental Material can be found at: /content/suppl/2020/12/18/73.1.202.DC1.html 1521-0081/73/1/202–277$35.00 https://doi.org/10.1124/pharmrev.120.000056 PHARMACOLOGICAL REVIEWS Pharmacol Rev 73:202–277, January 2021 Copyright © 2020 by The Author(s) This is an open access article distributed under the CC BY-NC Attribution 4.0 International license. ASSOCIATE EDITOR: MICHAEL NADER Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanismss Antonio Inserra, Danilo De Gregorio, and Gabriella Gobbi Neurobiological Psychiatry Unit, Department of Psychiatry, McGill University, Montreal, Quebec, Canada Abstract ...................................................................................205 Significance Statement. ..................................................................205 I. Introduction . ..............................................................................205 A. Review Outline ........................................................................205 B. Psychiatric Disorders and the Need for Novel Pharmacotherapies .......................206 C. Psychedelic Compounds as Novel Therapeutics in Psychiatry: Overview and Comparison with Current Available Treatments . .....................................206 D. Classical or Serotonergic Psychedelics versus Nonclassical Psychedelics: Definition ......208 Downloaded from E. Dissociative Anesthetics................................................................209 F. Empathogens-Entactogens . ............................................................209 -

Hyperlinked Region IX SOP for MWLCEMS
2 Region IX 2 0 0 STANDARD OPERATING PROCEDURES/ 1 1 STANDING MEDICAL ORDERS 9 9 McHenry Western Lake County EMS System Healthcare delivery requires structure (people, equipment, education) and process (policies, protocols, procedures) that, when integrated, produce a system (programs, organizations, cultures) that leads to optimal outcomes (patient survival and safety, quality, satisfaction). An effective system of care comprises all of these elements—structure, process, system, and patient outcomes—in a framework of continuous quality improvement (AHA, 2015). These protocols have been developed and approved through a collaborative process involving the Advocate Lutheran General; Greater Elgin Area, McHenry Western Lake County, Northwest Community, Amita Saint Joseph Hospital, and Southern Fox Valley EMS Systems to reduce variation in practice and establish a Region-wide System of care. They shall be used: as the written practice guidelines/pathways of care approved by the EMS Medical Directors (EMS MDs) to be initiated by System EMS personnel for off-line medical control. as the standing medical orders to be used by Emergency Communications Registered Nurses (ECRNs) when providing on-line medical control (OLMC). in medium to large scale multiple patient incidents, given that the usual and customary forms of communication are contraindicated as specified in the Region IX disaster plan. System members are authorized to implement these orders to their scope of practice. OLMC communication shall be established without endangering the patient. Under no circumstances shall emergency prehospital care be delayed while attempting to establish contact with a hospital. In the event that communications cannot be established, EMS personnel shall continue to provide care to the degree authorized by their license, these protocols, drugs/equipment available, and their scope of practice granted by the EMS MD in that System. -

Metered-Dose Inhalers (Mdis): Anti-Cholinergic Drugs
Texas Vendor Drug Program Drug Use Criteria: Aerosolized Agents - Metered-Dose Inhalers (MDIs): Anti-Cholinergic Drugs Publication History 1. Developed January 1995. 2. Revised April 2021; March 2019; March 2017; November 2015; March 2014; August 2012; June 2012; October 2010; January 2008; January 2003; January 2002; January 2001; March 2000; January 2000; February 1999; February 1998; February 1997; August 1995. Notes: All criteria may be applied retrospectively. The information contained is for the convenience of the public. The Texas Health and Human Services Commission is not responsible for any errors in transmission or any errors or omissions in the document. Medications listed in the tables and non-FDA approved indications included in these retrospective criteria are not indicative of Vendor Drug Program formulary coverage. Prepared by: • Drug Information Service, UT Health San Antonio. • The College of Pharmacy, The University of Texas at Austin 1 1 Dosage 1.1 Adults Ipratropium (Atrovent®), a short-acting, inhalational anticholinergic agent, is FDA- approved to manage bronchospasm associated with chronic bronchitis and emphysema, collectively known as chronic obstructive pulmonary disease (COPD). Ipratropium is considered a second-line agent in the treatment of asthma as the bronchodilatory effects seen with ipratropium are less than those seen with beta- adrenergic drugs. While not FDA approved, the Expert Panel 3 guidelines from the National Heart Lung and Blood Institute document benefit when multiple ipratropium doses are administered adjunctively with beta2-agonists in the emergency department to manage more severe acute asthma exacerbations, and the Global Initiative for Asthma (GINA) guidelines state that ipratropium may be considered an alternative bronchodilator in patients who experience adverse effects to short-acting beta2-agonists (e.g., tachycardia, arrhythmia, tremor). -
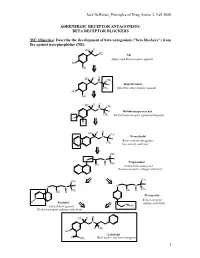
BETA RECEPTOR BLOCKERS MC Objective
Jack DeRuiter, Principles of Drug Action 2, Fall 2000 ADRENERGIC RECEPTOR ANTAGONISTS: BETA RECEPTOR BLOCKERS MC Objective: Describe the development of beta antagonists ("beta blockers") from the agonist norepinephrine (NE): HO H NH2 NE Alpha- and Beta-receptor agonist HO OH H H HO CH N 3 H Isoproterenol CH3 Selective beta-receptor agonist HO OH H H HO CH N 3 H Dichloroisoproterenol CH3 Partial beta-receptor agonist/antagonist Cl Cl H H HO CH N 3 Pronethalol H Beta-receptor antagonist, CH 3 low activity and toxic CH3 O N H Propranolol CH3 OH H Potent beta-antagonist No beta-receptor subtype selectivity CH3 CH3 O N H O N H CH CH OH H 3 OH H 3 Metoprolol N Beta-1-receptor H Pindolol O subtype selectivity Partial beta-agonist CH3 No beta-receptor subtype selectivity HO H H N H CH3 HO Labetolol O NH2 Dual alpha- and beta-antagonst 1 Jack DeRuiter, Principles of Drug Action 2, Fall 2000 MC Objective: Based on their structures, would the beta-blockers be expected to be relatively receptor selective? YES. They do not produce significant blockade of alpha- adrenergic receptors (alpha-1 or alpha-2), histamine receptors, muscarinic receptors or dopamine receptors. MC/PC Objective: Identify which beta blockers are classified as "non-selective": · The “non-selective" classification refers to those beta-blockers capable of blocking BOTH beta-1 and beta-2 receptors with equivalent efficacy. These drugs DO NOT have clinically significant affinity for other neurotransmitter receptors (alpha, dopamine, histamine, acetylcholine, etc.). · ALL of these beta-blockers (except satolol) consist of an aryloxypropanolamine side chain linked to an aromatic or “heteroaromatic” ring which is “ortho” substituted.