Bluegill Lepomis Macrochirus Synchronize Pectoral Fin Motion and Opercular Pumping
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bony Fish Guide
This guide will help you to complete the Bony Fish Observation Worksheet. Bony Fish Guide Fish (n.) An ectothermic (cold-blooded) vertebrate (with a backbone) aquatic (lives in water) animal that moves with the help of fins (limbs with no fingers or toes) and breathes with gills. This definition might seem very broad, and that is because fish are one of the most diverse groups of animals on the planet—there are a lot of fish in the sea (not to mention rivers, lakes and ponds). In fact, scientists count at least 32,000 species of fish—more than any other type of vertebrate. Fish are split into three broad classes: Jawless Fish Cartilaginous Fish Bony Fish (hagfish, lampreys, etc.) (sharks, rays, skates, etc.) (all other fish) This guide will focus on the Bony Fish. There are at least 28,000 species of bony fish, and they are found in almost every naturally occurring body of water on the planet. Bony fish range in size: • Largest: ocean sunfish (Mola mola), 11 feet, over 5,000 pounds • Smallest: dwarf pygmy goby (Pandaka pygmaea), ½ inch, a fraction of an ounce (This image is life size.) The following guide will help you learn more about the bony fish you can find throughout the New England Aquarium. Much of the guide is keyed to the Giant Ocean Tank, but can be applied to many kinds of fish. Even if you know nothing about fish, you can quickly learn a few things: The shape of a fish’s body, the position of its mouth and the shape of its tail can give you many clues as to its behavior and adaptations. -

Respiratory Disorders of Fish
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Disorders of the Respiratory System in Pet and Ornamental Fish a, b Helen E. Roberts, DVM *, Stephen A. Smith, DVM, PhD KEYWORDS Pet fish Ornamental fish Branchitis Gill Wet mount cytology Hypoxia Respiratory disorders Pathology Living in an aquatic environment where oxygen is in less supply and harder to extract than in a terrestrial one, fish have developed a respiratory system that is much more efficient than terrestrial vertebrates. The gills of fish are a unique organ system and serve several functions including respiration, osmoregulation, excretion of nitroge- nous wastes, and acid-base regulation.1 The gills are the primary site of oxygen exchange in fish and are in intimate contact with the aquatic environment. In most cases, the separation between the water and the tissues of the fish is only a few cell layers thick. Gills are a common target for assault by infectious and noninfectious disease processes.2 Nonlethal diagnostic biopsy of the gills can identify pathologic changes, provide samples for bacterial culture/identification/sensitivity testing, aid in fungal element identification, provide samples for viral testing, and provide parasitic organisms for identification.3–6 This diagnostic test is so important that it should be included as part of every diagnostic workup performed on a fish. -
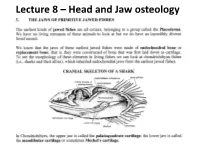
Lecture 8 – Head and Jaw Osteology
Lecture 8 – Head and Jaw osteology More derived fishes (Ray finned fishes) The variability of the jaw structure of bony fishes provides an explanation for the extensive adaptive radiation in the group and why they are so diverse and occupy almost every aquatic niche available. Skull diversity (A) carp, Cyprinus carpio, (B) vampire characin, Hydrolycus scomberoides, (C) catfish Arius felis. (D) cod Gadus morhua. (E) large-mouth bass, Micropterus salmoides (F) The parrotfish Scarus guacamaia. Scale bar = 10 mm WESTNEAT 2004 From an evolutionary standpoint, fishes were the first animals to develop bony jaws. Versatile jaws and multiple feeding strategies allowed fishes to fill, or radiate into, a diverse range of niches. They have evolved to feed in all possible ways – sucking, biting, scraping, nipping, crushing etc. The head of a teleost has 5 main regions: Cranium, jaws, cheeks, hydroid arch, opercula. The head of a fish has five main regions • 1) The CRANIUM is composed of the bones providing direct support and protection to the brain and the visual, Anterior Posterior olfactory, and auditory organs. Below the cranium is the parashenoid bone. • Parasphenoid plays a role in the jaws as Features of the neurocranium sensu lato (from Caranx it acts as a hard melampygus, lateral aspect, left, and posterior aspect, right). A = prevomer, B = ethmoid, C = frontal, D = palate supraoccipital, E = pterotic, F = exoccipital, G = basioccipital, H = foramen magnum, I = parasphenoid, J = orbit. The five main regions Bowfin 2) The JAWS • Lower Jaw – has an Angular articular and dentary bone • Angular articular- The paired bones form the posterior part of either side of the lower jaw and articulate with the suspensorium. -

Invasive Species of the Pacific Northwest
Invasive Species of the Pacific Northwest: Green Sunfish Lepomis cyanellus Derek Arterburn FISH 423: Olden 12.5.14 Figure 1: Adult Green sunfish Lepomis cyanellus . Photo from http://www.freshwater-fishing- news.com/fish-species-north -america/green-sunfish/ Classification Lepomis cyanellus may have a few teeth, Order: Perciformes which can be found on the tongue. Family: Centrarchidae Additional distinguishing marks are the 7-12 Genus: Lepomis parallel diffused dark bars running ventral to Species: cyanellus dorsal along the side of L. cyanellus, and the bluish-green pattern. The bluish-green Identification coloration takes place on the mainly black/dark brown/olive body, composed of Adult Green Sunfish, Lepomis ctenoid scales, which fades to a lighter cyanellus, commonly reach a total length of ventral color. The dark sides of L. cyanellus 31cm, with juveniles ranging from 12-15cm. are contrast with a yellow/cream ventral Adult Green Sunfish have been known to coloration (Cockerell 1913). The thick reach a maximum weight of one kilogram caudal peduncle is without an adipose fin, (2.2lbs). L. cyanellus is a deep bodied, and the peduncle runs to a rounded, slightly laterally compressed species, with a lateral forked, homocercal caudal fin. The paired line running from the operculum to the fins on Lepomis cyanellus are derived in caudal peduncle. The posterior of the orientation. The Green Sunfish has lateral operculum has a characteristic dark spot placement of the pectoral fins with vertical relatively the same size as the eye, and the insertion, anterior pelvic fins, and spines same size spot may also be found at the base found on the anal and dorsal fins. -

Percomorph Phylogeny: a Survey of Acanthomorphs and a New Proposal
BULLETIN OF MARINE SCIENCE, 52(1): 554-626, 1993 PERCOMORPH PHYLOGENY: A SURVEY OF ACANTHOMORPHS AND A NEW PROPOSAL G. David Johnson and Colin Patterson ABSTRACT The interrelationships of acanthomorph fishes are reviewed. We recognize seven mono- phyletic terminal taxa among acanthomorphs: Lampridiformes, Polymixiiformes, Paracan- thopterygii, Stephanoberyciformes, Beryciformes, Zeiformes, and a new taxon named Smeg- mamorpha. The Percomorpha, as currently constituted, are polyphyletic, and the Perciformes are probably paraphyletic. The smegmamorphs comprise five subgroups: Synbranchiformes (Synbranchoidei and Mastacembeloidei), Mugilomorpha (Mugiloidei), Elassomatidae (Elas- soma), Gasterosteiformes, and Atherinomorpha. Monophyly of Lampridiformes is justified elsewhere; we have found no new characters to substantiate the monophyly of Polymixi- iformes (which is not in doubt) or Paracanthopterygii. Stephanoberyciformes uniquely share a modification of the extrascapular, and Beryciformes a modification of the anterior part of the supraorbital and infraorbital sensory canals, here named Jakubowski's organ. Our Zei- formes excludes the Caproidae, and characters are proposed to justify the monophyly of the group in that restricted sense. The Smegmamorpha are thought to be monophyletic principally because of the configuration of the first vertebra and its intermuscular bone. Within the Smegmamorpha, the Atherinomorpha and Mugilomorpha are shown to be monophyletic elsewhere. Our Gasterosteiformes includes the syngnathoids and the Pegasiformes -

Anatomy and Go Fish! Background
Anatomy and Go Fish! Background Introduction It is important to properly identify fi sh for many reasons: to follow the rules and regulations, for protection against sharp teeth or protruding spines, for the safety of the fi sh, and for consumption or eating purposes. When identifying fi sh, scientists and anglers use specifi c vocabulary to describe external or outside body parts. These body parts are common to most fi sh. The difference in the body parts is what helps distinguish one fi sh from another, while their similarities are used to classify them into groups. There are approximately 29,000 fi sh species in the world. In order to identify each type of fi sh, scientists have grouped them according to their outside body parts, specifi cally the number and location of fi ns, and body shape. Classifi cation Using a system of classifi cation, scientists arrange all organisms into groups based on their similarities. The fi rst system of classifi cation was proposed in 1753 by Carolus Linnaeus. Linnaeus believed that each organism should have a binomial name, genus and species, with species being the smallest organization unit of life. Using Linnaeus’ system as a guide, scientists created a hierarchical system known as taxonomic classifi cation, in which organisms are classifi ed into groups based on their similarities. This hierarchical system moves from largest and most general to smallest and most specifi c: kingdom, phylum, class, order, family, genus, and species. {See Figure 1. Taxonomic Classifi cation Pyramid}. For example, fi sh belong to the kingdom Animalia, the phylum Chordata, and from there are grouped more specifi cally into several classes, orders, families, and thousands of genus and species. -

The Opercular Mouth-Opening Mechanism of Largemouth Bass Functions As a 3D Four-Bar Linkage with Three Degrees of Freedom Aaron M
© 2017. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2017) 220, 4612-4623 doi:10.1242/jeb.159079 RESEARCH ARTICLE The opercular mouth-opening mechanism of largemouth bass functions as a 3D four-bar linkage with three degrees of freedom Aaron M. Olsen1,*,‡,§, Ariel L. Camp2,‡ and Elizabeth L. Brainerd2 ABSTRACT (Greene, 1983), the thorax and wings of flying insects (Wootton, The planar, one degree of freedom (1-DoF) four-bar linkage is an 2009), and multiple skeletal elements in the skulls of fishes important model for understanding the function, performance and (Ballintijn, 1969; Westneat, 1990). In addition, four-bar models evolution of numerous biomechanical systems. One such system is have been used to evaluate functional hypotheses by comparing in vivo the opercular mechanism in fishes, which is thought to function like a simulated and kinematics (Westneat, 1991; Van four-bar linkage to depress the lower jaw. While anatomical and Wassenbergh et al., 2005; Roos et al., 2009), to measure how behavioral observations suggest some form of mechanical coupling, force and motion are transmitted through musculoskeletal systems previous attempts to model the opercular mechanism as a planar (Aerts and Verraes, 1984; Adriaens et al., 2001; Van Wassenbergh four-bar have consistently produced poor model fits relative to et al., 2013), and to examine the distribution and evolution of observed kinematics. Using newly developed, open source functional diversity (Westneat, 1995; Alfaro et al., 2004, 2005; mechanism fitting software, we fitted multiple three-dimensional Wainwright et al., 2004; Hulsey and García de León, 2005). (3D) four-bar models with varying DoF to in vivo kinematics in In biomechanical studies, four-bar models have been applied largemouth bass to test whether the opercular mechanism functions most extensively to the skulls of fishes. -

Fish Identification Guide Depicts More Than 50 Species of Fish Commonly Encoun- Make the Proper Identification of Every Fish Caught
he identification of different spe- Most species of fish are distinctive in appear- ance and relatively easy to identify. However, cies of fish has become an im- closely related species, such as members of the portant concern for recreational same “family” of fish, can present problems. For these species it is important to look for certain fishermen. The proliferation of T distinctive characteristics to make a positive regulations relating to minimum identification. sizes and possession limits compels fishermen to The ensuing fish identification guide depicts more than 50 species of fish commonly encoun- make the proper identification of every fish caught. tered in Virginia waters. In addition to color illustrations of each species, the description of each species lists the distinctive characteristics which enable a positive identification. Total Length FIRST DORSAL FIN Fork Length SECOND NUCHAL DORSAL FIN BAND SQUARE TAIL NARES FORKED TAIL GILL COVER (Operculum) CAUDAL LATRAL PEDUNCLE CHIN BARBELS LINE PECTORAL CAUDAL FIN ANAL FINS FIN PELVIC FINS GILL RAKERS GILL ARCH UNDERSIDE OF GILL COVER GILL RAKER GILL FILAMENTS GILL FILAMENTS DEFINITIONS Anal Fin – The fin on the bottom of fish located between GILL ARCHES 1st the anal vent (hole) and the tail. 2nd 3rd Barbels – Slender strands extending from the chins of 4th some fish (often appearing similar to whiskers) which per- form a sensory function. Caudal Fin – The tail fin of fish. Nuchal Band – A dark band extending from behind or Caudal Peduncle – The narrow portion of a fish’s body near the eye of a fish across the back of the neck toward immediately in front of the tail. -

Species Composition and Diversity of Fishes in the South China Sea, Area I: Gulf of Thailand and East Coast of Peninsular Malaysia
S4/FB3<CHAVALIT> Species composition and Diversity of Fishes in the South China Sea, Area I: Gulf of Thailand and East Coast of Peninsular Malaysia Chavalit Vidthayanon Department of Fisheries, Bangkok 10900, Thailand ABSTRACT The collaborative research on species composition and diversity of fishes in the Gulf of Thai- land and eastern Malay Peninsula was carried out by R. V. Pramong 4 in Thai waters and K.K. Manchong, K.K. Mersuji in Malaysian waters, through otter-board trawling surveys. Taxonomic surveys also done for commercial fishes in the markets of some localities. Totally 300 species from 18 orders and 89 families were obtained. Their diversity are drastically declined, compare to the previous survey from 380 species trawled. The station point of off Ko Chang, eastern Gulf of Thai- land and off Pahang River shown significantly high diversity of fishes57 and 73 species found. De- mersal species form the main composition of the catchs. The lizardfish Saurida undosquamis, S. miropectoralis, the bigeye Priacanthus tayenus and P. macracanthus, the rabbitfish Siganus canaliculatus and hairtail Trichiurus lepturus were the most abundant economic species found in mast of the sampling stations. Fishing efforts were 34 hours and 49 hours for the cruises I and II, with average catch per hour of 12.04 and 34.79 kg. respectively. The maximum catch per hour was 175.3 kg in Malaysian waters, the minimum was 4.33 kg in Thai waters. The average percentage of eco- nomic fishes is higher than that of trash fishes in Malaysian waters, it ranged from 55.45 to 81.92 %. -

NORTH COAST FISH IDENTIFICATION GUIDE Ben M
NORTH COAST FISH IDENTIFICATION GUIDE Ben M. Rome and Stephen J. Newman Department of Fisheries 3rd floor SGIO Atrium 168-170 St George’s Terrace PERTH WA 6000 Telephone (08) 9482 7333 Facsimile (08) 9482 7389 Website: www.fish.wa.gov.au ABN: 55 689 794 771 Published by Department of Fisheries, Perth, Western Australia. Fisheries Occasional Publications No. 80, September 2010. ISSN: 1447 - 2058 ISBN: 1 921258 90 X Information about this guide he intention of the North Coast Fish Identification Guide is to provide a simple, Teasy to use manual to assist commercial, recreational, charter and customary fishers to identify the most commonly caught marine finfish species in the North Coast Bioregion. This guide is not intended to be a comprehensive taxonomic fish ID guide for all species. It is anticipated that this guide will assist fishers in providing a more comprehensive species level description of their catch and hence assist scientists and managers in understanding any variation in the species composition of catches over both spatial and temporal scales. Fish taxonomy is a dynamic and evolving field. Advances in molecular analytical techniques are resolving many of the relationships and inter-relationships among species, genera and families of fishes. In this guide, we have used and adopted the latest taxonomic nomenclature. Any changes to fish taxonomy will be updated and revised in subsequent editions. The North Coast Bioregion extends from the Ashburton River near Onslow to the Northern Territory border. Within this region there is a diverse range of habitats from mangrove creeks, rivers, offshore islands, coral reef systems to continental shelf and slope waters. -

Fish Dissection Guide
Fish dissection guide Internal Anatomy: 1. Incision at anus . Begin by inserting a fine scalpel blade 4. Cut along isthmus . Use scissors to cut anteriorly through into the anus (also called the vent) of the fish. The anus is located the bones attached to the pelvic fins. Cut forward along the just anterior to (in front of) the anal fin, on the ventral (lower) side narrow, fleshy space beneath the head and between the gill of the fish in most fishes. covers. The gill covers (also known as operculae) are flaps which lie along both sides of the head and protect the underlying gills. 2. Cutting anteriorly. The incision is then extended anteriorly along the fish's belly towards the head. 5. Body cavity. Pull apart the two walls of the body cavity and expose the internal organs (see next image for names). The neat incision now runs from the anus forward between the two pelvic fins and along the isthmus. 3. Cut between pelvic fins . The incision passes anteriorly between the pelvic (ventral) fins. Depending on the type of fish, these paired fins are used to stabilise the fish when swimming and also for braking. The pelvic fins are supported by the bones of the . Some of the ventrally located internal pelvic girdle which are anchored in the belly muscles. 6. Internal organs organs: 1 heart, 2 Liver, 3 Pyloric caecae, 4 adipose (fatty) tissue 7. Pull aside gut. Here the adipose tissue (1) and gut (2) are 10. Swim bladder exposed. The other organs have been pulled aside to expose the swim bladder (3), gonads (4) and removed to expose the swim bladder at the top of the body kidneys (5). -

Understanding Fish Parts
The External Parts of the Fish and How They Help The Fish Grow and Move ACCESSING PRIOR KNOWLEDGE: Students share and the teacher records what students know and have experienced about fish. Encourage the discussion by asking questions such as: Ø What kind of environment do fish live in? Ø Who has fish at home? Ø How does a fish get its food? ACTIVITY #6: UNDERSTANDING FISH PARTS NGSS: K-ESS3-1, K-LS1-1 MATERIALS: • Diagram of the anatomy of a fish • Fish parts and their use- glossary • Fish to observe STEP 1: Students count and describe the fish in the fish tank. Students can describe the position of a fish in relationship to other objects using position words such as: above, below, behind, in front of, to the right/left of, etc. STEP 2: Students observe the fish in the tank and draw what they see. Students describe the parts they see and what they are used for. STEP 3: Students record their observations in their science notebook. EVALUATION: Depending on the grade level of the students, students will use a combination of drawing, dictating, and/or writing sentences or paragraphs to recall information, use facts and definitions, and provide a concluding statement. 1 2 3 4 5 6 7 ACTIVITY #2: HOW DO FISH GET OXYGEN? NGSS: MS-LS1-4 OBJECTIVE: Explain the basic method in which fish get oxygen, how they breathe underwater and understand the structures of the fish body that aide breathing and the exchange of oxygen and carbon dioxide. MATERIALS: Description of how fish get oxygen, teachers choice of materials for a sample model of how fish get oxygen.