Hydrodynamics of Suction Feeding in Fish
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

500 Natural Sciences and Mathematics
500 500 Natural sciences and mathematics Natural sciences: sciences that deal with matter and energy, or with objects and processes observable in nature Class here interdisciplinary works on natural and applied sciences Class natural history in 508. Class scientific principles of a subject with the subject, plus notation 01 from Table 1, e.g., scientific principles of photography 770.1 For government policy on science, see 338.9; for applied sciences, see 600 See Manual at 231.7 vs. 213, 500, 576.8; also at 338.9 vs. 352.7, 500; also at 500 vs. 001 SUMMARY 500.2–.8 [Physical sciences, space sciences, groups of people] 501–509 Standard subdivisions and natural history 510 Mathematics 520 Astronomy and allied sciences 530 Physics 540 Chemistry and allied sciences 550 Earth sciences 560 Paleontology 570 Biology 580 Plants 590 Animals .2 Physical sciences For astronomy and allied sciences, see 520; for physics, see 530; for chemistry and allied sciences, see 540; for earth sciences, see 550 .5 Space sciences For astronomy, see 520; for earth sciences in other worlds, see 550. For space sciences aspects of a specific subject, see the subject, plus notation 091 from Table 1, e.g., chemical reactions in space 541.390919 See Manual at 520 vs. 500.5, 523.1, 530.1, 919.9 .8 Groups of people Add to base number 500.8 the numbers following —08 in notation 081–089 from Table 1, e.g., women in science 500.82 501 Philosophy and theory Class scientific method as a general research technique in 001.4; class scientific method applied in the natural sciences in 507.2 502 Miscellany 577 502 Dewey Decimal Classification 502 .8 Auxiliary techniques and procedures; apparatus, equipment, materials Including microscopy; microscopes; interdisciplinary works on microscopy Class stereology with compound microscopes, stereology with electron microscopes in 502; class interdisciplinary works on photomicrography in 778.3 For manufacture of microscopes, see 681. -

Introduction
INTRODUCTION In nearly every body of water around the world, the most abundant vertebrate is a fi sh. From the deepest parts of the ocean to high alpine streams, fi shes live and reproduce, sometimes in places where no other vertebrates can survive. Whether peering out from a submarine while conducting deep-sea research, or stopping for a drink of water during a hike in the mountains, explorers, scientists, and naturalists fi nd fi shes. With well over 30,000 species, fi shes account for more than half of the total extant vertebrate diversity on Earth— in other words, there are more living species of fi shes than of amphibians, turtles, lizards, birds, and mammals combined. Not only are fi shes diverse in number of their species, but they are diverse in the habitats in which they live, the foods that they eat, the ways in which they reproduce, communicate, and interact with their environment, and the behaviors that they exhibit. Fishes can also be extremely abundant: the most abundant vertebrates on the planet are the small bristlemouth fi shes (Gonostomatidae) that are common throughout the vast open ocean. In some cases abundant fi shes such as cods, tunas, salmons, herrings, and anchovies support massive fi sheries that feed hundreds of millions of people. By supporting coastal communities and societies, these fi sheries (and the fi shes they target) have helped shape human history, becoming the foundation for coastal economies and an engine for global exploration and expansion. WHAT IS A FISH? Humans use the term “fi sh” to refer to several groups of vertebrates that do not have a clear set of diagnostic characteristics unique to them. -

Bony Fish Guide
This guide will help you to complete the Bony Fish Observation Worksheet. Bony Fish Guide Fish (n.) An ectothermic (cold-blooded) vertebrate (with a backbone) aquatic (lives in water) animal that moves with the help of fins (limbs with no fingers or toes) and breathes with gills. This definition might seem very broad, and that is because fish are one of the most diverse groups of animals on the planet—there are a lot of fish in the sea (not to mention rivers, lakes and ponds). In fact, scientists count at least 32,000 species of fish—more than any other type of vertebrate. Fish are split into three broad classes: Jawless Fish Cartilaginous Fish Bony Fish (hagfish, lampreys, etc.) (sharks, rays, skates, etc.) (all other fish) This guide will focus on the Bony Fish. There are at least 28,000 species of bony fish, and they are found in almost every naturally occurring body of water on the planet. Bony fish range in size: • Largest: ocean sunfish (Mola mola), 11 feet, over 5,000 pounds • Smallest: dwarf pygmy goby (Pandaka pygmaea), ½ inch, a fraction of an ounce (This image is life size.) The following guide will help you learn more about the bony fish you can find throughout the New England Aquarium. Much of the guide is keyed to the Giant Ocean Tank, but can be applied to many kinds of fish. Even if you know nothing about fish, you can quickly learn a few things: The shape of a fish’s body, the position of its mouth and the shape of its tail can give you many clues as to its behavior and adaptations. -

Respiratory Disorders of Fish
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Disorders of the Respiratory System in Pet and Ornamental Fish a, b Helen E. Roberts, DVM *, Stephen A. Smith, DVM, PhD KEYWORDS Pet fish Ornamental fish Branchitis Gill Wet mount cytology Hypoxia Respiratory disorders Pathology Living in an aquatic environment where oxygen is in less supply and harder to extract than in a terrestrial one, fish have developed a respiratory system that is much more efficient than terrestrial vertebrates. The gills of fish are a unique organ system and serve several functions including respiration, osmoregulation, excretion of nitroge- nous wastes, and acid-base regulation.1 The gills are the primary site of oxygen exchange in fish and are in intimate contact with the aquatic environment. In most cases, the separation between the water and the tissues of the fish is only a few cell layers thick. Gills are a common target for assault by infectious and noninfectious disease processes.2 Nonlethal diagnostic biopsy of the gills can identify pathologic changes, provide samples for bacterial culture/identification/sensitivity testing, aid in fungal element identification, provide samples for viral testing, and provide parasitic organisms for identification.3–6 This diagnostic test is so important that it should be included as part of every diagnostic workup performed on a fish. -

On Giant Filter Feeders 170 Million Years
PERSPECTIVES PALEONTOLOGY Massive fi lter-feeding vertebrates have roamed the world’s oceans for the past On Giant Filter Feeders 170 million years. Lionel Cavin he largest living marine verte- years ago), the diversity of mysticete whales these fi shes engulfed water by swimming brates—baleen whales and several was linked to the diversity of diatoms and to with an open mouth and sieved food while T lineages of sharks and rays—feed climatic variations (4 ). water escaped through the gill arches. directly on very small organisms (such as In the Jurassic (200 to 145 million years Giant reptiles roamed the Jurassic and plankton and small fi shes). Planktivorous ago) and the Cretaceous (145 to 65 million Cretaceous oceans, and some huge ray- sharks and rays collect food by fi ltering sea- years ago), ray-fi nned fi shes called pachy- fi nned fi shes—the ichthyodectiforms (bull- water through gill rakers (fi ngerlike pro- cormiforms lived in the oceans. These extinct dog fi sh and relatives)—emerged at the end jections on gill arches), whereas mysticete fishes are regarded as primitive teleosts, of the Cretaceous. But all these beasts were whales sieve small animals from seawater the group to which most living bony fi shes apex predators that fed on large preys, and through whalebone or baleen (comblike belong (5 ). A giant representative from the none had a fi lter-feeding diet. The newly keratin structures in their upper jaws) (1 , Middle Jurassic, Leedsichthys, was up to 9 discovered clade of massive fi lter-feeding 2). On page 990 of this issue, Friedman et m long and has been interpreted as a fi lter fi shes thus fi lls a large ecological niche. -

A Review of Planktivorous Fishes: Their Evolution, Feeding Behaviours, Selectivities, and Impacts
Hydrobiologia 146: 97-167 (1987) 97 0 Dr W. Junk Publishers, Dordrecht - Printed in the Netherlands A review of planktivorous fishes: Their evolution, feeding behaviours, selectivities, and impacts I Xavier Lazzaro ORSTOM (Institut Français de Recherche Scientifique pour le Développement eri Coopération), 213, rue Lu Fayette, 75480 Paris Cedex IO, France Present address: Laboratorio de Limrzologia, Centro de Recursos Hidricob e Ecologia Aplicada, Departamento de Hidraulica e Sarzeamento, Universidade de São Paulo, AV,DI: Carlos Botelho, 1465, São Carlos, Sï? 13560, Brazil t’ Mail address: CI? 337, São Carlos, SI? 13560, Brazil Keywords: planktivorous fish, feeding behaviours, feeding selectivities, electivity indices, fish-plankton interactions, predator-prey models Mots clés: poissons planctophages, comportements alimentaires, sélectivités alimentaires, indices d’électivité, interactions poissons-pltpcton, modèles prédateurs-proies I Résumé La vision classique des limnologistes fut de considérer les interactions cntre les composants des écosystè- mes lacustres comme un flux d’influence unidirectionnel des sels nutritifs vers le phytoplancton, le zoo- plancton, et finalement les poissons, par l’intermédiaire de processus de contrôle successivement physiqucs, chimiques, puis biologiques (StraSkraba, 1967). L‘effet exercé par les poissons plaiictophages sur les commu- nautés zoo- et phytoplanctoniques ne fut reconnu qu’à partir des travaux de HrbáEek et al. (1961), HrbAEek (1962), Brooks & Dodson (1965), et StraSkraba (1965). Ces auteurs montrèrent (1) que dans les étangs et lacs en présence de poissons planctophages prédateurs visuels. les conimuiiautés‘zooplanctoniques étaient com- posées d’espèces de plus petites tailles que celles présentes dans les milieux dépourvus de planctophages et, (2) que les communautés zooplanctoniques résultantes, composées d’espèces de petites tailles, influençaient les communautés phytoplanctoniques. -

Esox Lucius) Ecological Risk Screening Summary
Northern Pike (Esox lucius) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, February 2019 Web Version, 8/26/2019 Photo: Ryan Hagerty/USFWS. Public Domain – Government Work. Available: https://digitalmedia.fws.gov/digital/collection/natdiglib/id/26990/rec/22. (February 1, 2019). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2019a): “Circumpolar in fresh water. North America: Atlantic, Arctic, Pacific, Great Lakes, and Mississippi River basins from Labrador to Alaska and south to Pennsylvania and Nebraska, USA [Page and Burr 2011]. Eurasia: Caspian, Black, Baltic, White, Barents, Arctic, North and Aral Seas and Atlantic basins, southwest to Adour drainage; Mediterranean basin in Rhône drainage and northern Italy. Widely distributed in central Asia and Siberia easward [sic] to Anadyr drainage (Bering Sea basin). Historically absent from Iberian Peninsula, Mediterranean France, central Italy, southern and western Greece, eastern Adriatic basin, Iceland, western Norway and northern Scotland.” Froese and Pauly (2019a) list Esox lucius as native in Armenia, Azerbaijan, China, Georgia, Iran, Kazakhstan, Mongolia, Turkey, Turkmenistan, Uzbekistan, Albania, Austria, Belgium, Bosnia Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Macedonia, Moldova, Monaco, 1 Netherlands, Norway, Poland, Romania, Russia, Serbia, Slovakia, Slovenia, Sweden, Switzerland, United Kingdom, Ukraine, Canada, and the United States (including Alaska). From Froese and Pauly (2019a): “Occurs in Erqishi river and Ulungur lake [in China].” “Known from the Selenge drainage [in Mongolia] [Kottelat 2006].” “[In Turkey:] Known from the European Black Sea watersheds, Anatolian Black Sea watersheds, Central and Western Anatolian lake watersheds, and Gulf watersheds (Firat Nehri, Dicle Nehri). -
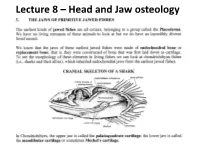
Lecture 8 – Head and Jaw Osteology
Lecture 8 – Head and Jaw osteology More derived fishes (Ray finned fishes) The variability of the jaw structure of bony fishes provides an explanation for the extensive adaptive radiation in the group and why they are so diverse and occupy almost every aquatic niche available. Skull diversity (A) carp, Cyprinus carpio, (B) vampire characin, Hydrolycus scomberoides, (C) catfish Arius felis. (D) cod Gadus morhua. (E) large-mouth bass, Micropterus salmoides (F) The parrotfish Scarus guacamaia. Scale bar = 10 mm WESTNEAT 2004 From an evolutionary standpoint, fishes were the first animals to develop bony jaws. Versatile jaws and multiple feeding strategies allowed fishes to fill, or radiate into, a diverse range of niches. They have evolved to feed in all possible ways – sucking, biting, scraping, nipping, crushing etc. The head of a teleost has 5 main regions: Cranium, jaws, cheeks, hydroid arch, opercula. The head of a fish has five main regions • 1) The CRANIUM is composed of the bones providing direct support and protection to the brain and the visual, Anterior Posterior olfactory, and auditory organs. Below the cranium is the parashenoid bone. • Parasphenoid plays a role in the jaws as Features of the neurocranium sensu lato (from Caranx it acts as a hard melampygus, lateral aspect, left, and posterior aspect, right). A = prevomer, B = ethmoid, C = frontal, D = palate supraoccipital, E = pterotic, F = exoccipital, G = basioccipital, H = foramen magnum, I = parasphenoid, J = orbit. The five main regions Bowfin 2) The JAWS • Lower Jaw – has an Angular articular and dentary bone • Angular articular- The paired bones form the posterior part of either side of the lower jaw and articulate with the suspensorium. -

Invasive Species of the Pacific Northwest
Invasive Species of the Pacific Northwest: Green Sunfish Lepomis cyanellus Derek Arterburn FISH 423: Olden 12.5.14 Figure 1: Adult Green sunfish Lepomis cyanellus . Photo from http://www.freshwater-fishing- news.com/fish-species-north -america/green-sunfish/ Classification Lepomis cyanellus may have a few teeth, Order: Perciformes which can be found on the tongue. Family: Centrarchidae Additional distinguishing marks are the 7-12 Genus: Lepomis parallel diffused dark bars running ventral to Species: cyanellus dorsal along the side of L. cyanellus, and the bluish-green pattern. The bluish-green Identification coloration takes place on the mainly black/dark brown/olive body, composed of Adult Green Sunfish, Lepomis ctenoid scales, which fades to a lighter cyanellus, commonly reach a total length of ventral color. The dark sides of L. cyanellus 31cm, with juveniles ranging from 12-15cm. are contrast with a yellow/cream ventral Adult Green Sunfish have been known to coloration (Cockerell 1913). The thick reach a maximum weight of one kilogram caudal peduncle is without an adipose fin, (2.2lbs). L. cyanellus is a deep bodied, and the peduncle runs to a rounded, slightly laterally compressed species, with a lateral forked, homocercal caudal fin. The paired line running from the operculum to the fins on Lepomis cyanellus are derived in caudal peduncle. The posterior of the orientation. The Green Sunfish has lateral operculum has a characteristic dark spot placement of the pectoral fins with vertical relatively the same size as the eye, and the insertion, anterior pelvic fins, and spines same size spot may also be found at the base found on the anal and dorsal fins. -

Percomorph Phylogeny: a Survey of Acanthomorphs and a New Proposal
BULLETIN OF MARINE SCIENCE, 52(1): 554-626, 1993 PERCOMORPH PHYLOGENY: A SURVEY OF ACANTHOMORPHS AND A NEW PROPOSAL G. David Johnson and Colin Patterson ABSTRACT The interrelationships of acanthomorph fishes are reviewed. We recognize seven mono- phyletic terminal taxa among acanthomorphs: Lampridiformes, Polymixiiformes, Paracan- thopterygii, Stephanoberyciformes, Beryciformes, Zeiformes, and a new taxon named Smeg- mamorpha. The Percomorpha, as currently constituted, are polyphyletic, and the Perciformes are probably paraphyletic. The smegmamorphs comprise five subgroups: Synbranchiformes (Synbranchoidei and Mastacembeloidei), Mugilomorpha (Mugiloidei), Elassomatidae (Elas- soma), Gasterosteiformes, and Atherinomorpha. Monophyly of Lampridiformes is justified elsewhere; we have found no new characters to substantiate the monophyly of Polymixi- iformes (which is not in doubt) or Paracanthopterygii. Stephanoberyciformes uniquely share a modification of the extrascapular, and Beryciformes a modification of the anterior part of the supraorbital and infraorbital sensory canals, here named Jakubowski's organ. Our Zei- formes excludes the Caproidae, and characters are proposed to justify the monophyly of the group in that restricted sense. The Smegmamorpha are thought to be monophyletic principally because of the configuration of the first vertebra and its intermuscular bone. Within the Smegmamorpha, the Atherinomorpha and Mugilomorpha are shown to be monophyletic elsewhere. Our Gasterosteiformes includes the syngnathoids and the Pegasiformes -

Anatomy and Go Fish! Background
Anatomy and Go Fish! Background Introduction It is important to properly identify fi sh for many reasons: to follow the rules and regulations, for protection against sharp teeth or protruding spines, for the safety of the fi sh, and for consumption or eating purposes. When identifying fi sh, scientists and anglers use specifi c vocabulary to describe external or outside body parts. These body parts are common to most fi sh. The difference in the body parts is what helps distinguish one fi sh from another, while their similarities are used to classify them into groups. There are approximately 29,000 fi sh species in the world. In order to identify each type of fi sh, scientists have grouped them according to their outside body parts, specifi cally the number and location of fi ns, and body shape. Classifi cation Using a system of classifi cation, scientists arrange all organisms into groups based on their similarities. The fi rst system of classifi cation was proposed in 1753 by Carolus Linnaeus. Linnaeus believed that each organism should have a binomial name, genus and species, with species being the smallest organization unit of life. Using Linnaeus’ system as a guide, scientists created a hierarchical system known as taxonomic classifi cation, in which organisms are classifi ed into groups based on their similarities. This hierarchical system moves from largest and most general to smallest and most specifi c: kingdom, phylum, class, order, family, genus, and species. {See Figure 1. Taxonomic Classifi cation Pyramid}. For example, fi sh belong to the kingdom Animalia, the phylum Chordata, and from there are grouped more specifi cally into several classes, orders, families, and thousands of genus and species. -

Filter-Feeding Ecology of Aquatic Insects
Annual Reviews www.annualreviews.org/aronline AnmRev. F.ntomol. 1980. 25:103-32 Copyright© 1980 by AnnualReviews Inc. All rights reserved FILTER-FEEDING ECOLOGY ~6185 OF AQUATIC INSECTS J. BruceWallace Departmentof Entomology,University of Georgia, Athens, Georgia 30602 Richard W. Merritt Departmentof Entomology,Michigan State University, East Lansing, Michigan48824 INTRODUCTION Filter feeders are organisms that have evolved various sieving mechanisms for removing particulate matter from suspension (100). Several groups aquatic insects, with habitats ranging from high elevation streams to saltwa- ter estuaries, use this feeding methodand consumesignificant quantities of suspended material (seston), including living organisms and both organic and inorganic detritus. Filter-feeding insects constitute important pathways for energy flow and are very important in the productivity of aquatic environments. Yet, someof these animals epitomize the complexrelation- ship between manand insects since biting adults of certain groups are amongman’s oldest adversaries. The major objectives of this article are to review the meansby which filter-feeding insects obtain their food and to assess the role of these animals in aquatic ecosystems.Filter-feeding strate- gies by other invertebrates in both marine and freshwater habitats have been partially reviewed elsewhere (82, 100, 101). SOURCES OF FOOD Lotic ecosystems in forested regions receive large inputs of allochthonous organic matter (4, 36-38, 98, 137). Anderson& Sedell (4) recently reviewed the role of macroinvertebrates in detritus processing. There is ample evi- dence from lotic studies that the concentration of particulate organic seston 103 0066-4170/80/0101-0103501.00 Annual Reviews www.annualreviews.org/aronline 104 WALLACE& MERRITT is skewed toward the smallest size fractions (<50 /zm) (119, 148, 191).