Whiting, Merlangius Merlangus.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Marine Fish Conservation Global Evidence for the Effects of Selected Interventions
Marine Fish Conservation Global evidence for the effects of selected interventions Natasha Taylor, Leo J. Clarke, Khatija Alliji, Chris Barrett, Rosslyn McIntyre, Rebecca0 K. Smith & William J. Sutherland CONSERVATION EVIDENCE SERIES SYNOPSES Marine Fish Conservation Global evidence for the effects of selected interventions Natasha Taylor, Leo J. Clarke, Khatija Alliji, Chris Barrett, Rosslyn McIntyre, Rebecca K. Smith and William J. Sutherland Conservation Evidence Series Synopses 1 Copyright © 2021 William J. Sutherland This work is licensed under a Creative Commons Attribution 4.0 International license (CC BY 4.0). This license allows you to share, copy, distribute and transmit the work; to adapt the work and to make commercial use of the work providing attribution is made to the authors (but not in any way that suggests that they endorse you or your use of the work). Attribution should include the following information: Taylor, N., Clarke, L.J., Alliji, K., Barrett, C., McIntyre, R., Smith, R.K., and Sutherland, W.J. (2021) Marine Fish Conservation: Global Evidence for the Effects of Selected Interventions. Synopses of Conservation Evidence Series. University of Cambridge, Cambridge, UK. Further details about CC BY licenses are available at https://creativecommons.org/licenses/by/4.0/ Cover image: Circling fish in the waters of the Halmahera Sea (Pacific Ocean) off the Raja Ampat Islands, Indonesia, by Leslie Burkhalter. Digital material and resources associated with this synopsis are available at https://www.conservationevidence.com/ -

Plastic and Evolutionary Responses to Climate Change in Fish
Evolutionary Applications Evolutionary Applications ISSN 1752-4571 REVIEWS AND SYNTHESIS Plastic and evolutionary responses to climate change in fish Lisa G. Crozier1 and Jeffrey A. Hutchings2,3 1 Northwest Fisheries Science Center, Seattle, WA, USA 2 Department of Biology, Dalhousie University, Halifax, NS, Canada 3 Department of Biosciences, Centre for Ecological and Evolutionary Synthesis, University of Oslo, Oslo, Norway Keywords Abstract adaptation, climate change, evolutionary theory, fisheries management, life-history The physical and ecological ‘fingerprints’ of anthropogenic climate change over evolution, phenotypic plasticity the past century are now well documented in many environments and taxa. We reviewed the evidence for phenotypic responses to recent climate change in fish. Correspondence Changes in the timing of migration and reproduction, age at maturity, age at Lisa G. Crozier, Northwest Fisheries Science juvenile migration, growth, survival and fecundity were associated primarily with Center, 2725 Montlake Blvd E., Seattle, WA changes in temperature. Although these traits can evolve rapidly, only two studies 98112, USA. Tel.: +1 206 860 3395 attributed phenotypic changes formally to evolutionary mechanisms. The correla- fax: +1 206 860 3267 tion-based methods most frequently employed point largely to ‘fine-grained’ e-mail: [email protected] population responses to environmental variability (i.e. rapid phenotypic changes relative to generation time), consistent with plastic mechanisms. Ultimately, Received: 10 May 2013 -

Bony Fish Guide
This guide will help you to complete the Bony Fish Observation Worksheet. Bony Fish Guide Fish (n.) An ectothermic (cold-blooded) vertebrate (with a backbone) aquatic (lives in water) animal that moves with the help of fins (limbs with no fingers or toes) and breathes with gills. This definition might seem very broad, and that is because fish are one of the most diverse groups of animals on the planet—there are a lot of fish in the sea (not to mention rivers, lakes and ponds). In fact, scientists count at least 32,000 species of fish—more than any other type of vertebrate. Fish are split into three broad classes: Jawless Fish Cartilaginous Fish Bony Fish (hagfish, lampreys, etc.) (sharks, rays, skates, etc.) (all other fish) This guide will focus on the Bony Fish. There are at least 28,000 species of bony fish, and they are found in almost every naturally occurring body of water on the planet. Bony fish range in size: • Largest: ocean sunfish (Mola mola), 11 feet, over 5,000 pounds • Smallest: dwarf pygmy goby (Pandaka pygmaea), ½ inch, a fraction of an ounce (This image is life size.) The following guide will help you learn more about the bony fish you can find throughout the New England Aquarium. Much of the guide is keyed to the Giant Ocean Tank, but can be applied to many kinds of fish. Even if you know nothing about fish, you can quickly learn a few things: The shape of a fish’s body, the position of its mouth and the shape of its tail can give you many clues as to its behavior and adaptations. -

Feeding Habits of the Common Thresher Shark (Alopias Vulpinus) Sampled from the California-Based Drift Gill Net Fishery, 1998-1 999
PRETI ET AL.: FEEDING HABITS OF COMMON THRESHER SHARK CalCOFl Rep., Vol. 42, 2001 FEEDING HABITS OF THE COMMON THRESHER SHARK (ALOPIAS VULPINUS) SAMPLED FROM THE CALIFORNIA-BASED DRIFT GILL NET FISHERY, 1998-1 999 ANTONELLA PRETI SUSAN E. SMITH AND DARLENE A. RAMON California Department of Fish and Game National Marine Fisheries Service, NOM 8604 La Jolla Shores Dnve Southwest Fisheries Science Center La Jolla, California 92037 P.O. Box 271 sharksharkshark@hotniail coni La Jolla, California 92038 ABSTRACT (Compagno 1984). It is epipelagic, gregarious, and cos- The diet of common thresher shark (Alopius vulpinus) mopolitan, and in the northeastern Pacific seems to be from US. Pacific Coast waters was investigated by means most abundant within 40 miles of shore (Strasburg 1958). of frequency of occurrence, gravimetric and numerical Its known range extends from Clarion Island, Mexico, methods, and calculating the geometric index of im- north to British Columbia; it is common seasonally from portance (GII) of prey taxa taken from stoniachs col- mid-Baja California, Mexico, to Washington state.' It lected by fishery observers from the California-based is the leading commercial shark taken in California, drift gill net fishery. Sampling was done from 16 August where it is highly valued in the fresh fish trade (Holts et 1998 to 24 January 1999, a time when the California al. 1998). It is also sought by recreational anglers for its Current was undergoing rapid change from El Niiio to fighting ability as well as food value, especially in south- La Niiia conhtions. Of the 165 stomachs examined, 107 ern California. -

Respiratory Disorders of Fish
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Disorders of the Respiratory System in Pet and Ornamental Fish a, b Helen E. Roberts, DVM *, Stephen A. Smith, DVM, PhD KEYWORDS Pet fish Ornamental fish Branchitis Gill Wet mount cytology Hypoxia Respiratory disorders Pathology Living in an aquatic environment where oxygen is in less supply and harder to extract than in a terrestrial one, fish have developed a respiratory system that is much more efficient than terrestrial vertebrates. The gills of fish are a unique organ system and serve several functions including respiration, osmoregulation, excretion of nitroge- nous wastes, and acid-base regulation.1 The gills are the primary site of oxygen exchange in fish and are in intimate contact with the aquatic environment. In most cases, the separation between the water and the tissues of the fish is only a few cell layers thick. Gills are a common target for assault by infectious and noninfectious disease processes.2 Nonlethal diagnostic biopsy of the gills can identify pathologic changes, provide samples for bacterial culture/identification/sensitivity testing, aid in fungal element identification, provide samples for viral testing, and provide parasitic organisms for identification.3–6 This diagnostic test is so important that it should be included as part of every diagnostic workup performed on a fish. -

Profiles of Whiting (Merlangius Merlangus Euxinus Nordman, 1840) Meat and Roe During Fishing Season in Black Sea
─── Food Technology ─── Comparison of fatty acids, lipid quality index and amino acid profiles of whiting (Merlangius merlangus euxinus Nordman, 1840) meat and roe during fishing season in Black Sea Demet Kocatepe, Can Okan Altan, Hülya Turan Sinop University, Sinop, Turkey Abstract Keywords: Introduction. The aim of research is to determine the fatty Whiting acid and amino acid composition of the whiting meat and roe Fish roe caught in different months in Black Sea. Amino acids Materials and methods. The whiting (Merlangius Fatty acids merlangus euxinus Nordman, 1840) caught from the Sinop EPA region of the Middle Black Sea Region. Sampling were carried DHA out twice a month. And the whiting meat and roe were compared in view of its fatty acid (FA) and amino acid (AA) composition during fishing season in Black Sea. Results and discussion. During the six months, the length and weight of whiting used in the study varied between 14.15– 16.60 cm and 24.49–29.68 g, respectively. Minimum length Article history: and weight were determined in March. Maximum crude protein values of fish meat and roes was determined in May Received 21.12.2018 Received in revised (18.61g/100g) and in April (16.30g/100g), respectively. SFA’s form 03.05.2019 (saturated fatty acids), MUFA’s (monounsaturated fatty acids) Accepted 30.09.2019 and PUFA’s (polyunsaturated fatty acids) of fish meat and roes had been varied during the season. The minimum and maximum EPA, DHA contents were found as 7.42–10.72, Corresponding 3.39–22.67g/100g in meat and 0.03-0.37, 3.79–4.76 g/100g in author: roe, respectively. -
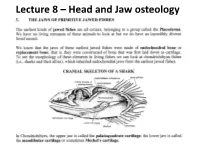
Lecture 8 – Head and Jaw Osteology
Lecture 8 – Head and Jaw osteology More derived fishes (Ray finned fishes) The variability of the jaw structure of bony fishes provides an explanation for the extensive adaptive radiation in the group and why they are so diverse and occupy almost every aquatic niche available. Skull diversity (A) carp, Cyprinus carpio, (B) vampire characin, Hydrolycus scomberoides, (C) catfish Arius felis. (D) cod Gadus morhua. (E) large-mouth bass, Micropterus salmoides (F) The parrotfish Scarus guacamaia. Scale bar = 10 mm WESTNEAT 2004 From an evolutionary standpoint, fishes were the first animals to develop bony jaws. Versatile jaws and multiple feeding strategies allowed fishes to fill, or radiate into, a diverse range of niches. They have evolved to feed in all possible ways – sucking, biting, scraping, nipping, crushing etc. The head of a teleost has 5 main regions: Cranium, jaws, cheeks, hydroid arch, opercula. The head of a fish has five main regions • 1) The CRANIUM is composed of the bones providing direct support and protection to the brain and the visual, Anterior Posterior olfactory, and auditory organs. Below the cranium is the parashenoid bone. • Parasphenoid plays a role in the jaws as Features of the neurocranium sensu lato (from Caranx it acts as a hard melampygus, lateral aspect, left, and posterior aspect, right). A = prevomer, B = ethmoid, C = frontal, D = palate supraoccipital, E = pterotic, F = exoccipital, G = basioccipital, H = foramen magnum, I = parasphenoid, J = orbit. The five main regions Bowfin 2) The JAWS • Lower Jaw – has an Angular articular and dentary bone • Angular articular- The paired bones form the posterior part of either side of the lower jaw and articulate with the suspensorium. -

Invasive Species of the Pacific Northwest
Invasive Species of the Pacific Northwest: Green Sunfish Lepomis cyanellus Derek Arterburn FISH 423: Olden 12.5.14 Figure 1: Adult Green sunfish Lepomis cyanellus . Photo from http://www.freshwater-fishing- news.com/fish-species-north -america/green-sunfish/ Classification Lepomis cyanellus may have a few teeth, Order: Perciformes which can be found on the tongue. Family: Centrarchidae Additional distinguishing marks are the 7-12 Genus: Lepomis parallel diffused dark bars running ventral to Species: cyanellus dorsal along the side of L. cyanellus, and the bluish-green pattern. The bluish-green Identification coloration takes place on the mainly black/dark brown/olive body, composed of Adult Green Sunfish, Lepomis ctenoid scales, which fades to a lighter cyanellus, commonly reach a total length of ventral color. The dark sides of L. cyanellus 31cm, with juveniles ranging from 12-15cm. are contrast with a yellow/cream ventral Adult Green Sunfish have been known to coloration (Cockerell 1913). The thick reach a maximum weight of one kilogram caudal peduncle is without an adipose fin, (2.2lbs). L. cyanellus is a deep bodied, and the peduncle runs to a rounded, slightly laterally compressed species, with a lateral forked, homocercal caudal fin. The paired line running from the operculum to the fins on Lepomis cyanellus are derived in caudal peduncle. The posterior of the orientation. The Green Sunfish has lateral operculum has a characteristic dark spot placement of the pectoral fins with vertical relatively the same size as the eye, and the insertion, anterior pelvic fins, and spines same size spot may also be found at the base found on the anal and dorsal fins. -

Percomorph Phylogeny: a Survey of Acanthomorphs and a New Proposal
BULLETIN OF MARINE SCIENCE, 52(1): 554-626, 1993 PERCOMORPH PHYLOGENY: A SURVEY OF ACANTHOMORPHS AND A NEW PROPOSAL G. David Johnson and Colin Patterson ABSTRACT The interrelationships of acanthomorph fishes are reviewed. We recognize seven mono- phyletic terminal taxa among acanthomorphs: Lampridiformes, Polymixiiformes, Paracan- thopterygii, Stephanoberyciformes, Beryciformes, Zeiformes, and a new taxon named Smeg- mamorpha. The Percomorpha, as currently constituted, are polyphyletic, and the Perciformes are probably paraphyletic. The smegmamorphs comprise five subgroups: Synbranchiformes (Synbranchoidei and Mastacembeloidei), Mugilomorpha (Mugiloidei), Elassomatidae (Elas- soma), Gasterosteiformes, and Atherinomorpha. Monophyly of Lampridiformes is justified elsewhere; we have found no new characters to substantiate the monophyly of Polymixi- iformes (which is not in doubt) or Paracanthopterygii. Stephanoberyciformes uniquely share a modification of the extrascapular, and Beryciformes a modification of the anterior part of the supraorbital and infraorbital sensory canals, here named Jakubowski's organ. Our Zei- formes excludes the Caproidae, and characters are proposed to justify the monophyly of the group in that restricted sense. The Smegmamorpha are thought to be monophyletic principally because of the configuration of the first vertebra and its intermuscular bone. Within the Smegmamorpha, the Atherinomorpha and Mugilomorpha are shown to be monophyletic elsewhere. Our Gasterosteiformes includes the syngnathoids and the Pegasiformes -

Anatomy and Go Fish! Background
Anatomy and Go Fish! Background Introduction It is important to properly identify fi sh for many reasons: to follow the rules and regulations, for protection against sharp teeth or protruding spines, for the safety of the fi sh, and for consumption or eating purposes. When identifying fi sh, scientists and anglers use specifi c vocabulary to describe external or outside body parts. These body parts are common to most fi sh. The difference in the body parts is what helps distinguish one fi sh from another, while their similarities are used to classify them into groups. There are approximately 29,000 fi sh species in the world. In order to identify each type of fi sh, scientists have grouped them according to their outside body parts, specifi cally the number and location of fi ns, and body shape. Classifi cation Using a system of classifi cation, scientists arrange all organisms into groups based on their similarities. The fi rst system of classifi cation was proposed in 1753 by Carolus Linnaeus. Linnaeus believed that each organism should have a binomial name, genus and species, with species being the smallest organization unit of life. Using Linnaeus’ system as a guide, scientists created a hierarchical system known as taxonomic classifi cation, in which organisms are classifi ed into groups based on their similarities. This hierarchical system moves from largest and most general to smallest and most specifi c: kingdom, phylum, class, order, family, genus, and species. {See Figure 1. Taxonomic Classifi cation Pyramid}. For example, fi sh belong to the kingdom Animalia, the phylum Chordata, and from there are grouped more specifi cally into several classes, orders, families, and thousands of genus and species. -

The Opercular Mouth-Opening Mechanism of Largemouth Bass Functions As a 3D Four-Bar Linkage with Three Degrees of Freedom Aaron M
© 2017. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2017) 220, 4612-4623 doi:10.1242/jeb.159079 RESEARCH ARTICLE The opercular mouth-opening mechanism of largemouth bass functions as a 3D four-bar linkage with three degrees of freedom Aaron M. Olsen1,*,‡,§, Ariel L. Camp2,‡ and Elizabeth L. Brainerd2 ABSTRACT (Greene, 1983), the thorax and wings of flying insects (Wootton, The planar, one degree of freedom (1-DoF) four-bar linkage is an 2009), and multiple skeletal elements in the skulls of fishes important model for understanding the function, performance and (Ballintijn, 1969; Westneat, 1990). In addition, four-bar models evolution of numerous biomechanical systems. One such system is have been used to evaluate functional hypotheses by comparing in vivo the opercular mechanism in fishes, which is thought to function like a simulated and kinematics (Westneat, 1991; Van four-bar linkage to depress the lower jaw. While anatomical and Wassenbergh et al., 2005; Roos et al., 2009), to measure how behavioral observations suggest some form of mechanical coupling, force and motion are transmitted through musculoskeletal systems previous attempts to model the opercular mechanism as a planar (Aerts and Verraes, 1984; Adriaens et al., 2001; Van Wassenbergh four-bar have consistently produced poor model fits relative to et al., 2013), and to examine the distribution and evolution of observed kinematics. Using newly developed, open source functional diversity (Westneat, 1995; Alfaro et al., 2004, 2005; mechanism fitting software, we fitted multiple three-dimensional Wainwright et al., 2004; Hulsey and García de León, 2005). (3D) four-bar models with varying DoF to in vivo kinematics in In biomechanical studies, four-bar models have been applied largemouth bass to test whether the opercular mechanism functions most extensively to the skulls of fishes. -

Little Fish, Big Impact: Managing a Crucial Link in Ocean Food Webs
little fish BIG IMPACT Managing a crucial link in ocean food webs A report from the Lenfest Forage Fish Task Force The Lenfest Ocean Program invests in scientific research on the environmental, economic, and social impacts of fishing, fisheries management, and aquaculture. Supported research projects result in peer-reviewed publications in leading scientific journals. The Program works with the scientists to ensure that research results are delivered effectively to decision makers and the public, who can take action based on the findings. The program was established in 2004 by the Lenfest Foundation and is managed by the Pew Charitable Trusts (www.lenfestocean.org, Twitter handle: @LenfestOcean). The Institute for Ocean Conservation Science (IOCS) is part of the Stony Brook University School of Marine and Atmospheric Sciences. It is dedicated to advancing ocean conservation through science. IOCS conducts world-class scientific research that increases knowledge about critical threats to oceans and their inhabitants, provides the foundation for smarter ocean policy, and establishes new frameworks for improved ocean conservation. Suggested citation: Pikitch, E., Boersma, P.D., Boyd, I.L., Conover, D.O., Cury, P., Essington, T., Heppell, S.S., Houde, E.D., Mangel, M., Pauly, D., Plagányi, É., Sainsbury, K., and Steneck, R.S. 2012. Little Fish, Big Impact: Managing a Crucial Link in Ocean Food Webs. Lenfest Ocean Program. Washington, DC. 108 pp. Cover photo illustration: shoal of forage fish (center), surrounded by (clockwise from top), humpback whale, Cape gannet, Steller sea lions, Atlantic puffins, sardines and black-legged kittiwake. Credits Cover (center) and title page: © Jason Pickering/SeaPics.com Banner, pages ii–1: © Brandon Cole Design: Janin/Cliff Design Inc.