Patterns of Evolution in the Feeding Mechanism of Actinopterygian Fishes1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogeny Classification Additional Readings Clupeomorpha and Ostariophysi
Teleostei - AccessScience from McGraw-Hill Education http://www.accessscience.com/content/teleostei/680400 (http://www.accessscience.com/) Article by: Boschung, Herbert Department of Biological Sciences, University of Alabama, Tuscaloosa, Alabama. Gardiner, Brian Linnean Society of London, Burlington House, Piccadilly, London, United Kingdom. Publication year: 2014 DOI: http://dx.doi.org/10.1036/1097-8542.680400 (http://dx.doi.org/10.1036/1097-8542.680400) Content Morphology Euteleostei Bibliography Phylogeny Classification Additional Readings Clupeomorpha and Ostariophysi The most recent group of actinopterygians (rayfin fishes), first appearing in the Upper Triassic (Fig. 1). About 26,840 species are contained within the Teleostei, accounting for more than half of all living vertebrates and over 96% of all living fishes. Teleosts comprise 517 families, of which 69 are extinct, leaving 448 extant families; of these, about 43% have no fossil record. See also: Actinopterygii (/content/actinopterygii/009100); Osteichthyes (/content/osteichthyes/478500) Fig. 1 Cladogram showing the relationships of the extant teleosts with the other extant actinopterygians. (J. S. Nelson, Fishes of the World, 4th ed., Wiley, New York, 2006) 1 of 9 10/7/2015 1:07 PM Teleostei - AccessScience from McGraw-Hill Education http://www.accessscience.com/content/teleostei/680400 Morphology Much of the evidence for teleost monophyly (evolving from a common ancestral form) and relationships comes from the caudal skeleton and concomitant acquisition of a homocercal tail (upper and lower lobes of the caudal fin are symmetrical). This type of tail primitively results from an ontogenetic fusion of centra (bodies of vertebrae) and the possession of paired bracing bones located bilaterally along the dorsal region of the caudal skeleton, derived ontogenetically from the neural arches (uroneurals) of the ural (tail) centra. -

The Adductor Pectoral Fin Muscle of Micropogonias Furnieri (Perciformes
ZOOLOGIA 33(6): e20160101 ISSN 1984-4689 (online) www.scielo.br/zool MORPHOLOGY AND PHYSIOLOGY The adductor pectoral fin muscle ofMicropogonias furnieri (Perciformes: Sciaenidae): a morphological and histochemical study María Sol Hernández1, María Victoria Longo1*, Clelia Viviana Devincenti1 & Alcira Ofelia Díaz1 1Instituto de Investigaciones Marinas y Costeras (IIMyC), Departamento de Biología, FCEyN, CONICET, Universidad Nacional de Mar del Plata. Funes 3250, 3° piso, (7600) Mar del Plata, Argentina. *Corresponding author. E-mail: [email protected] ABSTRACT. The adductor pectoral fin muscle in post-spawning females of the white croakerMicropogonias furnieri (Desmarest, 1823) is described from a morphological, histochemical and morphometric perspectives. A description of the morphological characteristics was conducted after dissections in different layers, down to the deep layer. Five muscles were found: superficial, medial, radial and deep adductors, and dorsal arrector. Their fibers were studied after applying histochemical techniques: succinic dehydrogenase (SDH) to reveal mitochondria, periodic acid-Schiff (PAS) for glycogen, Sudan black for lipids, myosin adenosinetriphosphatase (mATPase) to reveal the types of fibers, and modified mATPase to evidence the capillaries. Fiber diameters were measured and the number of capillaries was counted. Fiber subtypes named small, medium and large were found within red, pink and white fibers, the latter prevailing. Staining homogeneity was observed in white fibers after alkaline pre-incubations. The number of capillaries decreased from red to white fibers. Due to the prevalence of white fibers, the adductor muscle of the pectoral fins appears to be capable of rapid and discontinuous movements, which are important to body stabilization during subcarangiform swimming. The homogenous staining of white fibers observed in this research corresponds to the post-spawning gonad stage studied. -

Characterization of the G Protein-Coupled Receptor Family
www.nature.com/scientificreports OPEN Characterization of the G protein‑coupled receptor family SREB across fsh evolution Timothy S. Breton1*, William G. B. Sampson1, Benjamin Cliford2, Anyssa M. Phaneuf1, Ilze Smidt3, Tamera True1, Andrew R. Wilcox1, Taylor Lipscomb4,5, Casey Murray4 & Matthew A. DiMaggio4 The SREB (Super‑conserved Receptors Expressed in Brain) family of G protein‑coupled receptors is highly conserved across vertebrates and consists of three members: SREB1 (orphan receptor GPR27), SREB2 (GPR85), and SREB3 (GPR173). Ligands for these receptors are largely unknown or only recently identifed, and functions for all three are still beginning to be understood, including roles in glucose homeostasis, neurogenesis, and hypothalamic control of reproduction. In addition to the brain, all three are expressed in gonads, but relatively few studies have focused on this, especially in non‑mammalian models or in an integrated approach across the entire receptor family. The purpose of this study was to more fully characterize sreb genes in fsh, using comparative genomics and gonadal expression analyses in fve diverse ray‑fnned (Actinopterygii) species across evolution. Several unique characteristics were identifed in fsh, including: (1) a novel, fourth euteleost‑specifc gene (sreb3b or gpr173b) that likely emerged from a copy of sreb3 in a separate event after the teleost whole genome duplication, (2) sreb3a gene loss in Order Cyprinodontiformes, and (3) expression diferences between a gar species and teleosts. Overall, gonadal patterns suggested an important role for all sreb genes in teleost testicular development, while gar were characterized by greater ovarian expression that may refect similar roles to mammals. The novel sreb3b gene was also characterized by several unique features, including divergent but highly conserved amino acid positions, and elevated brain expression in pufer (Dichotomyctere nigroviridis) that more closely matched sreb2, not sreb3a. -

Ambloplites Constellatus, a New Species of Rock Bass from the Ozark Upland of Arkansas and Missouri with a Review of Western Rock Bass Populations
Ambloplites constellatus, a New Species of Rock Bass from the Ozark Upland of Arkansas and Missouri with a Review of Western Rock Bass Populations ROBERT C. CASHNER and ROYAL D. SUTTKUS V Reprinted from THE AMERICAN MIDLAND NATURALIST Vol. 98, No. 1, July, 1977, pp. 147-161 University of Notre Dame Press Notre Dame, Indiana Ambloplites constellatus, a New Species of Rock Bass from the Ozark Upland of Arkansas and Missouri with a Review of Western Rock Bass Populations' ROBERT C. CASHNER Department of Biological Sciences, University of New Orleans, New Orleans, Louisiana 70122 and ROYAL D. SUTTKUS Tulane University, Museum of Natural History, Belle Chasse, Louisiana 70037 ABSTRACT: A new species of rock bass, Ambloplites constellatus, is described from the upland section of the White River in Arkansas and Missouri. It is compared with the closely related northern rock bass (A. rupestris) from Missouri and Meramec river populations, the southern rock bass (A. ariommus) from the Ouachita and Little river drainages, and with other western rock bass populations of undetermined status. Ambloplites constellatus is distinguished from its congeners by its freckled color pattern and slender body form. Ambloplites constellatus occurs throughout the upper White River. There are two records of the species from the Osage River drainage in Missouri. INTRODUCTION In his study of Missouri fishes, Pflieger (1971) noted that rock bass from the upper White River system differed strikingly in color pattern from other Missouri populations. Based on our examination of mate- rial from throughout the Ozark Upland province, as well as other western rock bass populations, we describe the upper White River population as a new species, Ambloplites constellatus, the Ozark rock bass. -

Bony Fish Guide
This guide will help you to complete the Bony Fish Observation Worksheet. Bony Fish Guide Fish (n.) An ectothermic (cold-blooded) vertebrate (with a backbone) aquatic (lives in water) animal that moves with the help of fins (limbs with no fingers or toes) and breathes with gills. This definition might seem very broad, and that is because fish are one of the most diverse groups of animals on the planet—there are a lot of fish in the sea (not to mention rivers, lakes and ponds). In fact, scientists count at least 32,000 species of fish—more than any other type of vertebrate. Fish are split into three broad classes: Jawless Fish Cartilaginous Fish Bony Fish (hagfish, lampreys, etc.) (sharks, rays, skates, etc.) (all other fish) This guide will focus on the Bony Fish. There are at least 28,000 species of bony fish, and they are found in almost every naturally occurring body of water on the planet. Bony fish range in size: • Largest: ocean sunfish (Mola mola), 11 feet, over 5,000 pounds • Smallest: dwarf pygmy goby (Pandaka pygmaea), ½ inch, a fraction of an ounce (This image is life size.) The following guide will help you learn more about the bony fish you can find throughout the New England Aquarium. Much of the guide is keyed to the Giant Ocean Tank, but can be applied to many kinds of fish. Even if you know nothing about fish, you can quickly learn a few things: The shape of a fish’s body, the position of its mouth and the shape of its tail can give you many clues as to its behavior and adaptations. -

Respiratory Disorders of Fish
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Disorders of the Respiratory System in Pet and Ornamental Fish a, b Helen E. Roberts, DVM *, Stephen A. Smith, DVM, PhD KEYWORDS Pet fish Ornamental fish Branchitis Gill Wet mount cytology Hypoxia Respiratory disorders Pathology Living in an aquatic environment where oxygen is in less supply and harder to extract than in a terrestrial one, fish have developed a respiratory system that is much more efficient than terrestrial vertebrates. The gills of fish are a unique organ system and serve several functions including respiration, osmoregulation, excretion of nitroge- nous wastes, and acid-base regulation.1 The gills are the primary site of oxygen exchange in fish and are in intimate contact with the aquatic environment. In most cases, the separation between the water and the tissues of the fish is only a few cell layers thick. Gills are a common target for assault by infectious and noninfectious disease processes.2 Nonlethal diagnostic biopsy of the gills can identify pathologic changes, provide samples for bacterial culture/identification/sensitivity testing, aid in fungal element identification, provide samples for viral testing, and provide parasitic organisms for identification.3–6 This diagnostic test is so important that it should be included as part of every diagnostic workup performed on a fish. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Carboniferous Formations and Faunas of Central Montana
Carboniferous Formations and Faunas of Central Montana GEOLOGICAL SURVEY PROFESSIONAL PAPER 348 Carboniferous Formations and Faunas of Central Montana By W. H. EASTON GEOLOGICAL SURVEY PROFESSIONAL PAPER 348 A study of the stratigraphic and ecologic associa tions and significance offossils from the Big Snowy group of Mississippian and Pennsylvanian rocks UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1962 UNITED STATES DEPARTMENT OF THE INTERIOR STEWART L. UDALL, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director The U.S. Geological Survey Library has cataloged this publication as follows : Eastern, William Heyden, 1916- Carboniferous formations and faunas of central Montana. Washington, U.S. Govt. Print. Off., 1961. iv, 126 p. illus., diagrs., tables. 29 cm. (U.S. Geological Survey. Professional paper 348) Part of illustrative matter folded in pocket. Bibliography: p. 101-108. 1. Paleontology Montana. 2. Paleontology Carboniferous. 3. Geology, Stratigraphic Carboniferous. I. Title. (Series) For sale by the Superintendent of Documents, U.S. Government Printing Office Washington 25, B.C. CONTENTS Page Page Abstract-__________________________________________ 1 Faunal analysis Continued Introduction _______________________________________ 1 Faunal relations ______________________________ 22 Purposes of the study_ __________________________ 1 Long-ranging elements...__________________ 22 Organization of present work___ __________________ 3 Elements of Mississippian affinity.._________ 22 Acknowledgments--.-------.- ___________________ -

Summary Report of Freshwater Nonindigenous Aquatic Species in U.S
Summary Report of Freshwater Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 4—An Update April 2013 Prepared by: Pam L. Fuller, Amy J. Benson, and Matthew J. Cannister U.S. Geological Survey Southeast Ecological Science Center Gainesville, Florida Prepared for: U.S. Fish and Wildlife Service Southeast Region Atlanta, Georgia Cover Photos: Silver Carp, Hypophthalmichthys molitrix – Auburn University Giant Applesnail, Pomacea maculata – David Knott Straightedge Crayfish, Procambarus hayi – U.S. Forest Service i Table of Contents Table of Contents ...................................................................................................................................... ii List of Figures ............................................................................................................................................ v List of Tables ............................................................................................................................................ vi INTRODUCTION ............................................................................................................................................. 1 Overview of Region 4 Introductions Since 2000 ....................................................................................... 1 Format of Species Accounts ...................................................................................................................... 2 Explanation of Maps ................................................................................................................................ -
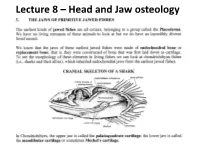
Lecture 8 – Head and Jaw Osteology
Lecture 8 – Head and Jaw osteology More derived fishes (Ray finned fishes) The variability of the jaw structure of bony fishes provides an explanation for the extensive adaptive radiation in the group and why they are so diverse and occupy almost every aquatic niche available. Skull diversity (A) carp, Cyprinus carpio, (B) vampire characin, Hydrolycus scomberoides, (C) catfish Arius felis. (D) cod Gadus morhua. (E) large-mouth bass, Micropterus salmoides (F) The parrotfish Scarus guacamaia. Scale bar = 10 mm WESTNEAT 2004 From an evolutionary standpoint, fishes were the first animals to develop bony jaws. Versatile jaws and multiple feeding strategies allowed fishes to fill, or radiate into, a diverse range of niches. They have evolved to feed in all possible ways – sucking, biting, scraping, nipping, crushing etc. The head of a teleost has 5 main regions: Cranium, jaws, cheeks, hydroid arch, opercula. The head of a fish has five main regions • 1) The CRANIUM is composed of the bones providing direct support and protection to the brain and the visual, Anterior Posterior olfactory, and auditory organs. Below the cranium is the parashenoid bone. • Parasphenoid plays a role in the jaws as Features of the neurocranium sensu lato (from Caranx it acts as a hard melampygus, lateral aspect, left, and posterior aspect, right). A = prevomer, B = ethmoid, C = frontal, D = palate supraoccipital, E = pterotic, F = exoccipital, G = basioccipital, H = foramen magnum, I = parasphenoid, J = orbit. The five main regions Bowfin 2) The JAWS • Lower Jaw – has an Angular articular and dentary bone • Angular articular- The paired bones form the posterior part of either side of the lower jaw and articulate with the suspensorium. -

Developmental Genetic Modularity of Cichlid Fish Dentitions
This is a repository copy of Biting into the Genome to Phenome Map: Developmental Genetic Modularity of Cichlid Fish Dentitions. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/109900/ Version: Accepted Version Article: Hulsey, C.D., Fraser, G.J. orcid.org/0000-0002-7376-0962 and Meyer, A. (2016) Biting into the Genome to Phenome Map: Developmental Genetic Modularity of Cichlid Fish Dentitions. Integrative and Comparative Biology, 56 (3). pp. 373-388. ISSN 1540-7063 https://doi.org/10.1093/icb/icw059 This is a pre-copyedited, author-produced version of an article accepted for publication in Integrative and Comparative Biology following peer review. The version of record Integr. Comp. Biol. (2016) 56 (3): 373-388 is available online at: https://doi.org/10.1093/icb/icw059. Reuse Unless indicated otherwise, fulltext items are protected by copyright with all rights reserved. The copyright exception in section 29 of the Copyright, Designs and Patents Act 1988 allows the making of a single copy solely for the purpose of non-commercial research or private study within the limits of fair dealing. The publisher or other rights-holder may allow further reproduction and re-use of this version - refer to the White Rose Research Online record for this item. Where records identify the publisher as the copyright holder, users can verify any specific terms of use on the publisher’s website. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. -

5. the Pesciara-Monte Postale Fossil-Lagerstätte: 2. Fishes and Other Vertebrates
Rendiconti della Società Paleontologica Italiana, 4, 2014, pp. 37-63 Excursion guidebook CBEP 2014-EPPC 2014-EAVP 2014-Taphos 2014 Conferences The Bolca Fossil-Lagerstätten: A window into the Eocene World (editors C.A. Papazzoni, L. Giusberti, G. Carnevale, G. Roghi, D. Bassi & R. Zorzin) 5. The Pesciara-Monte Postale Fossil-Lagerstätte: 2. Fishes and other vertebrates [ CARNEVALE, } F. BANNIKOV, [ MARRAMÀ, ^ C. TYLER & ? ZORZIN G. Carnevale, Dipartimento di Scienze della Terra, Università degli Studi di Torino, Via Valperga Caluso 35, I-10125 Torino, Italy; [email protected] A.F. Bannikov, Borisyak Paleontological Institute, Russian Academy of Sciences, Profsoyuznaya 123, Moscow 117997, Russia; [email protected] G. Marramà, Dipartimento di Scienze della Terra, Università degli Studi di Torino, Via Valperga Caluso, 35 I-10125 Torino, Italy; [email protected] J.C. Tyler, National Museum of Natural History, Smithsonian Institution (MRC-159), Washington, D.C. 20560 USA; [email protected] R. Zorzin, Sezione di Geologia e Paleontologia, Museo Civico di Storia Naturale di Verona, Lungadige Porta Vittoria 9, I-37129 Verona, Italy; [email protected] INTRODUCTION ][` ~[~ `[ =5} =!+~ [=5~5 Ceratoichthys pinnatiformis5 #] ~}==5[ ~== }}=OP[~` [ "O**""P "}[~* "+5$!+? 5`=5` ~]!5`5 =5=[~5_ O"!P#! [=~=55~5 `#~! ![[[~= O"]!#P5`` `5} 37 G. Carnevale, A.F. Bannikov, G. Marramà, J.C. Tyler & R. Zorzin FIG. 1_Ceratoichthys pinnatiformis~=5"!Q5=` 5. The Pesciara-Monte Postale Fossil-Lagerstätte: 2. Fishes and other vertebrates `== `]5"`5`" O*!P[~ `= =5<=[ ~#_5` [#5!="[ [~OQ5=5""="P5 ` [~`}= =5^^+55 ]"5++"5"5* *5 [=5` _5 [==5 *5]5[=[[5* [5=~[` +~++5~5=!5 ["5#+?5?5[=~[+" `[+=\`` 5`55`_= [~===5[=[5 ```_`5 [~5+~++5 [}5` `=5} 5= [~5O# "~++[=[+ P5`5 ~[O#P #"5[+~` [=Q5 5" QRQ5$5 ][5**~= [`OQ= RP`=5[` `+5=+5`=` +5 _O# P5+5 O? ]P _ #`[5[=~ [+#+?5` !5+`}==~ `5``= "!=Q5 "`O? ]P+5 _5`~[ =`5= G.