Shattering Glass Cookware Sur- Efaced As an Issue of Concern During the Past Two Decades, and Reports of Problems Have Been Chron- Icled in Several News Stories
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tevilas Keilim
STAR-K Kosher Classroom HALACHOS OF TEVIFOOD UTENSIL TEVILA GUIDELINELASFOOD UTENSIL TEVILAKEILIM GUIDELINE FOOD UTENSIL TEVILA GUIDELINE Earthenware, Non-Glazed Porcelain Enamel Tevila w/o Brocha Aluminum Pans, Disposable Tevila with Brocha No Tevila to be used more than once Dull Finish, e.g. Flower Pot Racks, Cooling Tevila w/o Brocha George Foreman Grill Tevila w/o Brocha Aluminum Pans, Disposable Tevila w/o Brocha Racks, Oven No Tevila to be used only once (including Pyrex, Duralex & Corelle) Glass Tevila with Brocha Rolling Pins Metal or Wood No Tevila Blech No Tevila Hot Air Popcorn Maker, Metal Tevila with Brocha Sandwich Maker Tevila w/o Brocha Blender -Handheld Immersion Hot Water Urn, Metal Tevila with Brocha Wand with metal blade on bottom Tevila with Brocha Sink Racks, Stainless Steel No Tevila Ice Cream Scooper Tevila with Brocha Bone No Tevila Spatula, Metal Tevila with Brocha Knife, Arts & Crafts No Tevila Brush, Pastry No Tevila Stoneware Tevila w/o Brocha Knife Sharpener No Tevila Brush for Grill, Metal No Tevila Stoneware, Non-Glazed No Tevila Meat Thermometer No Tevila Can Opener No Tevila Storage Utensils, Glass Meat Tenderizer Hammer, not brought to the table No Tevila Cans, Reusable Empty Metal No Tevila if opened by a Yehudi No Tevila Metal Cutlery Tevila with Brocha Styrofoam No Tevila China, Bone Tevila w/o Brocha Tea Kettle, Corelle Tevila with Brocha Metal Flour & Sugar Storage Tevila w/o Brocha China, Glazed Tevila w/o Brocha Canisters Thermos (Glass Insert) Tevila with Brocha Colander, Metal Tevila with Brocha -
Catalog Holding List A-E in PDF Form
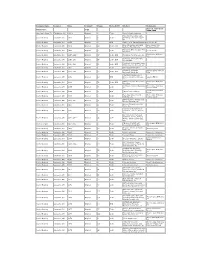
Company Name Location Date Original? Pages Color-B/W? Product Comments See U.S. Glass Circa Adams & Co. Copy 1890-1900 Akro Agate Glass Co. Clarksburg, WV 1940’s Original 4 Color Akroite floral containers Decorated beverage ware, Anchor Hocking Lancaster, OH 1942 Original 11 Color Fire-King oven ware & jade- ite Anchor Hocking Lancaster, OH 1953 Original 4 B/W Cape Cod or Sandwich pattern Loose sheets Decorated glass, Sandwich Some pages have Anchor Hocking Lancaster, OH 1954 Original 44 Color, B/W pattern, Fire-King, etc. pictures cut out Turquoise Blue & Copper Tint Anchor Hocking Lancaster, OH 1956 Original 5 Color Loose sheets Fire-King Plus 4 page B/W price Anchor Hocking Lancaster, OH 1957-1958 Original 47 Color, B/W Fire-King, Forest Green, etc. list Fire-King, beverage items, Anchor Hocking Lancaster, OH 1959-1960 Original 56 Color, B/W kitchenware Fire-King, kitchenware, some Anchor Hocking Lancaster, OH 1960-1961 Original 59 Color, B/W Early American Pres-cut, etc. Anchor Hocking Lancaster, OH 1961 Original 1 Color Desert gold stemware Fire-King, Sandwich, Several pages with cut Anchor Hocking Lancaster, OH 1961-1962 Original 67 Color, B/W beverage items, etc. outs “Institutional Glassware”, Anchor Hocking Lancaster, OH 1963 Original 12 B/W Stems, tumblers, Ashtrays, Catalog HR 63 etc. Mostly beverage ware, some Plus 8 page B/W price Anchor Hocking Lancaster, OH 1964 Original 78 Color, B/W Fire-King list Tumblers, Prescut, Ovenware, Plus 2 page B/W price Anchor Hocking Lancaster, OH 1965 Original 78 Color etc. revision sheet Loose sheet, includes Anchor Hocking Lancaster, OH 1965 Original 2 B/W “Bravo” Cut Tumblers prices Beverage Ware, Prescut, , Plus 1 page B/W price Anchor Hocking Lancaster, OH 1966 Original 80 Color Jade-ite, etc. -

Glossary of Terms Abrisa Technologies Your Single Source Optics Partner!
Glossary of Terms Abrisa Technologies Your Single Source Optics Partner! December 2015 200 South Hallock Drive, Santa Paula, CA 93060 • (877) 622-7472 • FAX (805) 525-8604 • www.abrisatechnologies.com Glossary of Terms - 12/15 2 of 13 Acid Etching This process for the decoration of glass involves the application of hydrofluoric acid to the glass surface. Hydrofluoric acid vapors or baths of hydrofluoric acid salts may be used to give glass a matte, frosted appearance (similar to that obtained by surface sandblasting), as found in lighting glass. Glass designs can be produced by coating the glass with wax and then inscribing the desired pattern through the wax layer. When applied, the acid will corrode the glass but not attack the wax-covered areas. Alumina-silicate Glass Alumina (aluminum oxide Al2O3) is added to the glass batch in the form of commonly found feldspars containing alkalis in order to help improve chemical resistance and mechanical strength, and to increase viscosity at lower temperatures. Angle of Incidence The angle formed between a ray of light striking a surface and the normal line (the line perpendicular to the surface at that point). Annealing Under natural conditions, the surface of molten glass will cool more rapidly than the center. This results in internal stress- es which may cause the glass sheet or object to crack, shatter or even explode some time later. The annealing process is designed to eliminate or limit such stresses by submitting the glass to strictly controlled cooling in a special oven known as a “lehr”. Inside the lehr, the glass is allowed to cool to a temperature known as the “annealing point”. -

The Pennsylvania State University
The Pennsylvania State University The Graduate School ADVENTURES IN HIGH DIMENSIONS: UNDERSTANDING GLASS FOR THE 21ST CENTURY A Dissertation in Material Science and Engineering by Collin James Wilkinson © 2021 Collin James Wilkinson Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy May 2021 ii The dissertation of Collin Wilkinson was reviewed and approved by the following: John Mauro Professor of Materials Science and Engineering Chair, Intercollege Graduate Degree Program Associate Head for Graduate Education, Materials Science and Engineering Dissertation Advisor Chair of Committee Seong Kim Professor of Chemical Engineering Professor of Materials Science and Engineering Ismaila Dabo Associate Professor of Materials Science and Engineering Susan Sinnott Professor of Materials Science and Engineering Professor of Chemistry Head of the Department of Materials Science and Engineering iii Abstract Glass is infinitely variable. This complexity stands as a promising technology for the 21st century since the need for environmentally friendly materials has reached a critical point due to climate change. However, such a wide range of variability makes new glass compositions difficult to design. The difficulty is only exaggerated when considering that not only is there an infinite variability in the compositional space, but also an infinite variability thermal history of a glass and in the crystallinity of glass-cearmics. This means that even for a simple binary glass there are at least 3 dimensions that have to be optimized. To resolve this difficulty, it is shown that energy landscapes can capture all three sets of complexity (composition, thermal history, and crystallinity). The explicit energy landscape optimization, however, has a large computational cost. -

Mead Art Museum Andrew W. Mellon Faculty Seminar: Jan 15 and 16, 2015
Mead Art Museum Andrew W. Mellon Faculty Seminar: Jan 15 and 16, 2015 Looking at Glass through an Interdisciplinary Lens: Teaching and Learning with the Mead’s Collection Books: Bach, Hans and Norbert Neuroth, eds. The Properties of Optical Glass. Berlin: Springer-Verlag, 1995. Barr, Sheldon. Venetian Glass: Confections in Glass, 1855-1914. New York: Harry N. Abrams, 1998. Battie, David and Simon Cottle, eds. Sotheby's Concise Encyclopedia of Glass. London: Conran Octopus, 1991. Blaszczyk, Regina Lee. Imagining Consumers, Design and Innovation from Wedgwood to Corning. Baltimore: Johns Hopkins University Press, 2000. Bradbury, S. The Evolution of the Microscope. Oxford: Pergamon Press, 1967. Busch, Jason T., and Catherine L. Futter. Inventing the Modern World: Decorative Arts at the World’s Fairs, 1951-1939. New York, NY: Skira Rizzoli, 2012. Carboni, Stefano and Whitehouse, David. Glass of the Sultans. New York: Metropolitan Museum of Art; Corning, NY: The Corning Museum of Glass; Athens: Benaki Museum; New Haven and London: Yale University Press, 2001. Charleston, Robert J. Masterpieces of glass: a world history from the Corning Museum of Glass. 2nd ed.: New York, Harry N. Abrams, 1990. The Corning Museum of Glass. Innovations in Glass. Corning, New York: The Corning Museum of Glass, 1999. Lois Sherr Dubin. The History of Beads: from 30,000 B.C. to the present. London: Thames & Hudson, 2006. Fleming, Stuart. Roman Glass: Reflections of Everyday Life. Philadelphia: University of Pennsylvania Museum, 1997. ----Roman Glass: Reflections on Cultural Change. Philadelphia: University of Pennsylvania Museum of Archaeology and Anthropology, 1999. 1 Frelinghuysen, Alice Cooney. Louis Comfort Tiffany at the Metropolitan Museum. -

New Glass Review 10.Pdf
'New Glass Review 10J iGl eview 10 . The Corning Museum of Glass NewG lass Review 10 The Corning Museum of Glass Corning, New York 1989 Objects reproduced in this annual review Objekte, die in dieser jahrlich erscheinenden were chosen with the understanding Zeitschrift veroffentlicht werden, wurden unter that they were designed and made within der Voraussetzung ausgewahlt, dal3 sie the 1988 calendar year. innerhalb des Kalenderjahres 1988 entworfen und gefertigt wurden. For additional copies of New Glass Review, Zusatzliche Exemplare des New Glass Review please contact: konnen angefordert werden bei: The Corning Museum of Glass Sales Department One Museum Way Corning, New York 14830-2253 (607) 937-5371 All rights reserved, 1989 Alle Rechtevorbehalten, 1989 The Corning Museum of Glass The Corning Museum of Glass Corning, New York 14830-2253 Corning, New York 14830-2253 Printed in Dusseldorf FRG Gedruckt in Dusseldorf, Bundesrepublik Deutschland Standard Book Number 0-87290-119-X ISSN: 0275-469X Library of Congress Catalog Card Number Aufgefuhrt im Katalog der KongreB-Bucherei 81-641214 unter der Nummer 81-641214 Table of Contents/lnhalt Page/Seite Jury Statements/Statements der Jury 4 Artists and Objects/Kunstler und Objekte 10 Bibliography/Bibliographie 30 A Selective Index of Proper Names and Places/ Verzeichnis der Eigennamen und Orte 53 er Wunsch zu verallgemeinern scheint fast ebenso stark ausgepragt Jury Statements Dzu sein wie der Wunsch sich fortzupflanzen. Jeder mochte wissen, welchen Weg zeitgenossisches Glas geht, wie es in der Kunstwelt bewer- tet wird und welche Stile, Techniken und Lander maBgeblich oder im Ruckgang begriffen sind. Jedesmal, wenn ich mich hinsetze und einen Jurybericht fur New Glass Review schreibe (dies ist mein 13.), winden he desire to generalize must be almost as strong as the desire to und krummen sich meine Gedanken, um aus den tausend und mehr Dias, Tprocreate. -

Vitrification of Historic and Future High Level Nuclear Wastes Within Alkali Borosilicate Glasses
Vitrification of historic and future high level nuclear wastes within alkali borosilicate glasses Andrew James Connelly M.Eng. A Thesis submitted to the Department of Engineering Materials at the University of Sheffield in partial fulfilment of the requirement for the Degree of Doctor of Philosophy. February 2008 The University Of Sheffield. Abstract The disposal of highly radioactive and toxic wastes generated by the nuclear industry is one of the biggest challenges facing the world today. Currently, in the UK there is a large legacy waste holding which has been accumulating since nuclear energy was first harnessed during World War 2. Processing of this waste with a view to final disposal is a complex and difficult task. This work investigates one aspect of that process, namely turning this waste into glass (or vitrification). This work uses multiple techniques including x-ray absorption spectroscopy, magic angle spinning nuclear magnetic resonance and molecular dynamic simulations, to investigate the structural role of Zr02 and U 03 within the alkali borosilicate glass used in the UK for waste immobilisation. The effect of these additions on the bulk glass structure and selected glass properties are also explored. In waste glasses Zr occurs as a 6 co-ordinated Zr ion surrounded by Si, B, Na and Li. The effect of Zr02 additions on the bulk glass structure and properties is highly complex. The addition of Zr02 appears to be characterised by a non-linearity in the trends of certain physical and structural parameters. At low levels of Zr02 the level of leaching from the glasses and the co ordination of B increase. -

Technical Glasses
Technical Glasses Physical and Technical Properties 2 SCHOTT is an international technology group with 130 years of ex perience in the areas of specialty glasses and materials and advanced technologies. With our highquality products and intelligent solutions, we contribute to our customers’ success and make SCHOTT part of everyone’s life. For 130 years, SCHOTT has been shaping the future of glass technol ogy. The Otto Schott Research Center in Mainz is one of the world’s leading glass research institutions. With our development center in Duryea, Pennsylvania (USA), and technical support centers in Asia, North America and Europe, we are present in close proximity to our customers around the globe. 3 Foreword Apart from its application in optics, glass as a technical ma SCHOTT Technical Glasses offers pertinent information in terial has exerted a formative influence on the development concise form. It contains general information for the deter of important technological fields such as chemistry, pharma mination and evaluation of important glass properties and ceutics, automotive, optics, optoelectronics and information also informs about specific chemical and physical character technology. Traditional areas of technical application for istics and possible applications of the commercial technical glass, such as laboratory apparatuses, flat panel displays and glasses produced by SCHOTT. With this brochure, we hope light sources with their various requirements on chemical to assist scientists, engineers, and designers in making the physical properties, have led to the development of a great appropriate choice and make optimum use of SCHOTT variety of special glass types. Through new fields of appli products. cation, particularly in optoelectronics, this variety of glass types and their modes of application have been continually Users should keep in mind that the curves or sets of curves enhanced, and new forming processes have been devel shown in the diagrams are not based on precision measure oped. -

Borosilicate Glass Wafers Are an Inexpensive Alternative
Basics of Microstructuring 01 Chapter MicroChemicals® – Fundamentals of Microstructuring www.microchemicals.com/downloads/application_notes.html PRODUCTION AND SPECIFICATIONS OF GLASS WAFERS For applications where neither the high dielectric strength of quartz nor the high transparency is in the ultravi- olet, visible or infrared spectral range or the thermal stability of quartz or quartz glass is required, borosilicate glass wafers are an inexpensive alternative. Borosilicate Glass and Ordinary Glass in Comparison Composition Borosilicate glass consists of approximately 80% of silicon dioxide (SiO2) and approximately 5-15% boron trioxide (B2O3). Other additives are alkaline oxide (Na2O, K2O), aluminium oxide (Al2O3) and alkaline po- tassium oxide (CaO, MgO). Borosilicate glasses typically have a very low iron content, which causes the typical green colour of window glass in order to increase the transparency. Properties Due to its boron content, borosilicate glass exhibit a higher chemical stability against water, many chemi- cals and pharmaceutical products compared to window glass. In addition, borosilicate glass exhibits signifi cantly higher thermal stability against temperature fl uctua- tions due to its thermal expansion coeffi cients, which are less than half as large as compared to window glass. Production of Glass Wafers For the further processing into glass wafers, specifi c borosilicate glass is produced using the “fl oat pro- cess” (Fig. 37). Hereby the molten glass on melted tin forms a fl oating both-side smooth ribbon with a homogeneous thickness. On its way on the tin bath, the temperature is gradually reduced from 1100 down to 600°C until the sheet can be lifted onto rollers. Glass pane Glass melt Tin melt Fig. -

High-Precision Micro-Machining of Glass for Mass-Personalization and Submitted in Partial Fulfillment of the Requirements for the Degree Of
High-precision micro-machining of glass for mass-personalization Lucas Abia Hof A Thesis In the Department of Mechanical, Industrial and Aerospace Engineering Presented in Partial Fulfillment of the Requirements For the Degree of Doctor of Philosophy (Mechanical Engineering) at Concordia University Montreal, Québec, Canada June 2018 © Lucas Abia Hof, 2018 CONCORDIA UNIVERSITY School of Graduate Studies This is to certify that the thesis prepared By: Lucas Abia Hof Entitled: High-precision micro-machining of glass for mass-personalization and submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Mechanical Engineering) complies with the regulations of the University and meets the accepted standards with respect to originality and quality. Signed by the final examining committee: ______________________________________ Chair Dr. K. Schmitt ______________________________________ External Examiner Dr. P. Koshy ______________________________________ External to Program Dr. M. Nokken ______________________________________ Examiner Dr. C. Moreau ______________________________________ Examiner Dr. R. Sedaghati ______________________________________ Thesis Supervisor Dr. R. Wüthrich Approved by: ___________________________________________________ Dr. A. Dolatabadi, Graduate Program Director August 14, 2018 __________________________________________________ Dr. A. Asif, Dean Faculty of Engineering and Computer Science Abstract High-precision micro-machining of glass for mass- personalization Lucas Abia Hof, -

The Bewildering Array of Owens-Illinois Glass Co. Logos and Codes
The Bewildering Array of Owens-Illinois Glass Co. Logos and Codes Bill Lockhart and Russ Hoenig [In 2004, Lockhart wrote similar articles about maker’s marks and codes used by the Owens-Illinois Glass Co. for the Society for Historical Archaeology Newsletter and a collectors’ magazine. While these articles contained more detailed information than earlier works (e.g., Toulouse 1971), there were still problems with some of the identifications. The current study corrects the issues and explains past discrepancies. Parts of this study were taken from Lockhart 2007 (online version of 2004 article).] From its beginning in 1929, the Owens-Illinois Glass Co. has been a giant in the bottle and jar industry. Its history (see below) is filled with growth and innovation. As a result, there is probably no way to even estimate the billions of bottles that Owens-Illinois has produced during the more than 80 years of its tenure. That means, of course, that the Owens-Illinois manufacturer’s mark and codes are the most common of all logos found by historical archaeologists in excavations and surveys of post 1930 sites. The study of these marks and codes are therefore of great interest to archaeologists studying material culture. Bottle collectors were the first group outside of the bottle industry to be introduced to the Owens-Illinois codes via a letter from Julian Harrison Toulouse to May Jones, published in Volume 5 of The Bottle Trail (1965). Toulouse served his entire career as an employee of Owens-Illinois, and, as his retirement neared, he wrote numerous articles and two books aimed at bottle collectors. -

Corning's Care and Safe Handling of Glassware Application Note
Care and Safe Handling of Laboratory Glassware Care and Safe Handling of Laboratory Glassware CONTENTS Glass: The Invisible Container . 1 Glass Technical Data . 2 PYREX ® Glassware . 2 PYREXPLUS ® Glassware . 2 PYREX Low Actinic Glassware . 2 VYCOR ® Glassware . 2 Suggestions for Safe Use of PYREX Glassware . 3 Safely Using Chemicals . 3 Safely Handling Glassware . 3 Heating and Cooling . 4 Autoclaving . 4 Mixing and Stirring . 5 Using Stopcocks . 5 Joining and Separating Glass Apparatus . 5 Using Rubber Stoppers . 6 Vacuum Applications . 6 Suggestions for Safe Use of PYREXPLUS Glassware . 6 Exposure to Heat . 7 Exposure to Cold . 7 Exposure to Chemicals . 7 Exposure to Ultraviolet . 7 Exposure to Microwave . 7 Exposure to Vacuum . 7 Autoclaving . 7 Labeling and Marking . 8 Suggestions for Safe Use of Fritted Glassware . 8 Selecting Fritted Glassware . 8 Proper Care of Fritted Ware . 8 Suggestions for Safe Use of Volumetric Glassware . 9 Types of Volumetric Glassware . 9 Calibrated Glassware Markings . 9 Reading Volumetric Glassware . 9 Suggestions for Cleaning and Storing Glassware . 10 Safety Considerations . 10 Cleaning PYREX Glassware . 10 Cleaning PYREXPLUS Glassware . 12 Cleaning Cell Culture Glassware . 12 Rinsing, Drying and Storing Glassware . 13 Glass Terminology . 13 Care and Safe Handling of Laboratory Glassware GLASS: THE INVISIBLE MATERIAL Q PYREX glassware comes in a wide variety of laboratory shapes, sizes and degrees of accuracy — a design to meet From the 16th century to today, chemical researchers have used every experimental need. glass containers for a very basic reason: the glass container is transparent, almost invisible and so its contents and reactions While we feel PYREX laboratory glassware is the best all- within it are clearly visible.