Structural and Functional Characteristics of Two Institutional Research Ethics Committees in Eswatini
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prospectus 2021/2022
THE UNIVERSITY OF ESWATINI 2021 PROSPECTUS FOR UNDERGRADUATE PROGRAMMES Admissions Office University of Eswatini February 2021 Thank you for your enquiry about the University of Eswatini. The aim of the booklet is to provide information about: The University and services it offers. The Undergraduate Study Programmes The Admission Requirements The Application Procedure Please note that the information contained in this booklet was correct at the time of going to print but may be changed without notice. Please address correspondence to: The Registrar Attention: Admissions Office University of Eswatini Private Bag 4 KWALUSENI M201 Or Email us at [email protected] 1 BACKGROUND INFORMATION Historical Note The University Of Eswatini (UNESWA) developed from the University of Botswana, Lesotho and Eswatini (UBLS), formerly known as the University of Basutoland, Bechuanaland and Swaziland (UBBS), which had its headquarters in Lesotho between 1964 and 1975. The UBBS had developed from the Pius XII Catholic University College at Roma – so our history has quite deep roots. UNESWA achieved its independent status as a fully-fledged University in 1982. Since achieving university status, UNESWA has continued to grow and to develop in accordance with its stated aim of assisting national development. Student enrolment in accordance has risen steadily, paralleled by an ever-increasing output of graduates since the University’s first Congregation for the conferment of Degrees in 1982. In all 20545 degrees have been conferred, 1156 of them at the 2019 Graduation. The chief mandate, which the university has tried to implement, is human resource production. This is clearly indicated in the type of programmes selected at the beginning, which still constitute a major part of UNESWA programmes. -

Scientific African
SCIENTIFIC AFRICAN AUTHOR INFORMATION PACK TABLE OF CONTENTS XXX . • Description p.1 • Abstracting and Indexing p.1 • Editorial Board p.1 • Guide for Authors p.6 ISSN: 2468-2276 DESCRIPTION . Scientific African is a peer reviewed, open access, inter- and multidisciplinary scientific journal that is dedicated to expanding access to African research, increasing intra-African scientific collaboration, and building academic research capacity in Africa. The journal aims to provide a modern, highly-visible platform for publishing pan-African research and welcomes submissions from all scientific disciplines in the following broad categories: AGF - Agriculture and Food Security CHE - Chemistry CON - Conservation and Sustainability Studies ECO - Economics and Business ENV - Environmental and Geosciences ITE - Information Technology and Engineering LIF - Life and Health Sciences MAT - Mathematics PHY - Physical Sciences SOC - Social Sciences and Policy The journal welcomes submissions of full text research articles, reviews but also publishes invited perspectives and critical policy papers. ABSTRACTING AND INDEXING . Directory of Open Access Journals (DOAJ) Emerging Sources Citation Index (ESCI) Scopus INSPEC EDITORIAL BOARD . Editor-in-Chief Benji Gyampoh, Kwame Nkrumah University of Science and Technology Department of Fisheries and Watershed Management, Kumasi, Ghana AUTHOR INFORMATION PACK 24 Sep 2021 www.elsevier.com/locate/sciaf 1 Editors Agriculture and Food Security Robert C. Abaidoo, Kwame Nkrumah University of Science and Technology, -

About the Contributors
ABOUT THE CONTRIBUTORS Michael Cross began his career as lecturer at the Faculty of Education, University of the Witwatersrand in 1986. He has been awarded teaching and research fellowships in several institutions including the Johns Hopkins University and North- western University. He was a visiting scholar at Stanford University, Stockholm University and Jules-Vernes University in Amiens. Winner of the 1911–12 award as most Outstanding Mentor of Educational Researchers in Africa from the Association for Educational Development in Africa (ADEA), Professor Cross is author and co- author of several books, book chapters and numerous articles in leading scholarly journals. He has served as an education specialist in several major national education policy initiatives in South Africa, such as the National Commission on Higher Education and the Technical Committee on Norms and Standards for Educators. He is currently the Director of the Ali Mazrui Centre for Higher Education Studies at the University of Johannesburg. James Otieno Jowi is the founding Executive Director/Secretary General of the African Network for Internationalization of Education (ANIE), an African network focused on the international dimension of higher education in Africa. He heads the ANIE Secretariat based at Moi University, Kenya, and is responsible for the implementation of ANIE activities. He also teaches Comparative and International Education at the School of Education, Moi University. He has published on internationalization of higher education, governance, management and leadership in higher education. He was member of the IAU Task Force on the 3rd and 4th Global Surveys on Internationalization of Higher Education. He is also a member of the IAU Ad-hoc Expert Group on Rethinking Internationalization. -

Gender Center and Gender Mainstreaming
Gender Center and Gender Mainstreaming Educational level: University | Beneficiaries: Students, faculty, and staff Background Assessments of universities such as Jimma University1 and the University of Dar es Salaam (UDSM)2 that found sexual harassment and violence and high attrition of female students played a role in developing gender centers.1 At the University of Western Cape, campus activism on issues including gender imbalances in salary and career development, sexual harassment, and maternity leave and child care contributed to the creation of a gender center.3 In other institutions, national and institutional commitment was key. For example, one of the objectives of the Presidential Working Party to establish Moi University was to develop a gender center, and the university’s 2005-2014 strategic plan committed to incorporating gender issues in policy decision-making processes.4 Makerere University also enjoyed a supportive national legislative environment in Uganda.5 Description Many institutions, including Jimma University, Moi University, UDSM, and Makerere University, note the role of the gender centers in promoting gender mainstreaming. The gender centers, offices, and committees at the institutions included in this review shared some common functions, including gender equality-related policy development, provision of training, skills-building, mentoring, counseling services, networking, information sharing, and research. Some institutions also provide scholarships to female students (Jimma University,6 Makerere University,5 University of Toronto7); facilitate housing for female faculty (Jimma University,6 University of Western Cape3); develop curricula on gender-related issues (the University of Ghana8); and develop proposals for “gender sensitive infrastructure within the University”9 (Sokoine University of Agriculture). The University of Toronto has multiple offices that work on diversity and equity issues. -
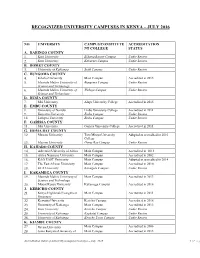
Recognized University Campuses in Kenya – July 2016
RECOGNIZED UNIVERSITY CAMPUSES IN KENYA – JULY 2016 NO. UNIVERSITY CAMPUS/CONSTITUTE ACCREDITATION NT COLLEGE STATUS A. BARINGO COUNTY 1. Kisii University Eldama Ravine Campus Under Review 2. Kisii University Kabarnet Campus Under Review B. BOMET COUNTY 3. University of Kabianga Sotik Campus Under Review C. BUNGOMA COUNTY 4. Kibabii University Main Campus Accredited in 2015 5. Masinde Muliro University of Bungoma Campus Under Review Science and Technology 6. Masinde Muliro University of Webuye Campus Under Review Science and Technology D. BUSIA COUNTY 7. Moi University Alupe University College Accredited in 2015 E. EMBU COUNTY 8. University of Nairobi Embu University College Accredited in 2011 9. Kenyatta University Embu Campus Under Review 10. Laikipia University Embu Campus Under Review F. GARISSA COUNTY 11. Moi University Garissa University College Accredited in 2011 G. HOMA BAY COUNTY 12. Maseno University Tom Mboya University Adopted as accredited in 2016 College 13. Maseno University Homa Bay Campus Under Review H. KAJIADO COUNTY 14. Adventist University of Africa Main Campus Accredited in 2013 15. Africa Nazarene University Main Campus Accredited in 2002 16. KAG EAST University Main Campus Adopted as accredited in 2014 17. The East African University Main Campus Accredited in 2010 18. KCA University Kitengela Campus Under Review I. KAKAMEGA COUNTY 19. Masinde Muliro University of Main Campus Accredited in 2013 Science and Technology 20. Mount Kenya University Kakamega Campus Accredited in 2016 J. KERICHO COUNTY 21. Kenya Highlands Evangelical Main Campus Accredited in 2011 University 22. Kenyatta University Kericho Campus Accredited in 2016 23. University of Kabianga Main Campus Accredited in 2013 24. -

Registered Participant List
AORTIC 2019 Registered Participant List ~As of 1 November 2019~ First Name Last Name Organisation Country Kunuz Abdella American Cancer Society Ethiopia Fatimah Abdulkareem College of Medicine University of Lagos Nigeria Habiba Ibrahim Abdullahi University of Abuja Teaching Hospital Nigeria Christian Abnet National Cancer Institute United States Natasha Abraham National Cancer Registry South Africa Mustapha Abubakar National Cancer Institute United States Dafalla Omer Elmustafa Abuidris Gezira University Sudan Emmah Achieng AMPATH Kenya Esther Serwaa Ackah Roche Products Ghana Ghana Briony Ackroyd Malawi College of Medicine United Kingdom Abdullahi Adamu Ahmadu Bello University Teaching Hospital Nigeria Danladi Adamu Gombe State University | University of Edinburgh United Kingdom Adebola Adedimeji Albert Einstein College of Medicine United States Babatunde Adedokun University of Chicago United States Ayodeji Adefemi Lagos State University Teaching Hospital Nigeria Bolanle Comfort Adegboyega Lagos University Teaching Hospital Nigeria Prisca Adejumo University of Ibadan Nigeria Taiwo Adegbola Adejuyigbe Dynamicgoogleintr Nigeria Adenike Adeniji-Sofoluwe University of Ibadan Nigeria Henry Adeola University of Cape Town South Africa Adekunle Adesina Baylor College of Medicine United States Margaret Adhiambo AIC Kijabe Hospital Kenya Amalia Adler-Waxman Teva Pharmaceuticals Israel Kasimu Adoke Government Nigeria Ben Adusei 37 Military Hospital Ghana Melhaoui Adyl Centre National de Réhabilitation Morocco Ilir Agalliu Albert Einstein College -

Medical School Celebrates 79 Young Doctors
UBOfficial UniversityNEWS of Botswana Newsletter www.ub.bw September 2018 MEDICAL SCHOOL CELEBRATES 79 YOUNG DOCTORS he University of Botswana School of country as well as to be kind, respectful and respect and to honour work and the profession’s TMedicine celebrated the 5th cohort of 79 caring to their clients. ethics. young doctors who graduated with Bachelor Professor Sebudubudu also advised them Ms Maphorisa said their contribution was Degrees in Medicine and Surgery (MBBS) with to always abide by their professional ethical very essential in assisting government and a dinner at the Phakalane Golf Estate Resort in code of conduct and to be part of the solutions the ministry to be more progressive. She also August. instead of the problem. In addition, he urged advised the young doctors to be cautious when Speaking at the Inaugural Convocation them to take their internship training seriously dealing with clients because some of them may Dinner for the Class of 2018, Dean of the Faculty and to face challenges in their profession head not necessarily present clinical deficiencies but of Medicine, Dr Oatlhokwa Nkomazana, said of on. social issues that needed to be referred to other the number, 69 graduated from the University “Make use of the internship training professionals. of Botswana while the rest were from abroad. opportunity and invest in your curriculum The Permanent Secretary also advised the Dr Nkomazana said the young doctors would vitae,” said Professor David Sebudubudu. graduates to be part of team work and study go for internship in public hospitals. Since its He also urged the graduates to further the environment where they would be engaged inception in 2009, the Medical School has so their studies at the University of Botswana as well as to be vigilant with their mental far graduated 200 doctors and most of them Medical School because the University was well health to avoid conditions such as depression. -

Meeting Report
The International AIDS Society Educational Fund meeting Outcome report 26-27 March 2019 Esulwini, Kingdom of Eswatini Science, Community and Youth in the HIV Response in Southern Africa 1 Table of Contents 1. LIST OF ABBREVIATIONS AND ACRONYMS ............................................................................................... 3 2. INTRODUCTION ........................................................................................................................................ 6 3. BACKGROUND AND CONTEXT .................................................................................................................. 7 4. MEETING REPORT .................................................................................................................................... 9 4.1 EXECUTIVE SUMMARY ......................................................................................................................................... 9 4.2 OFFICIAL OPENING AND WELCOME ADDRESSES ..................................................................................................... 10 5. KEY MESSAGES FROM AIDS 2018 ........................................................................................................... 13 5.1 HIV PREVENTION AND TREATMENT UPDATES - PROF KENNETH NGURE, IAS GOVERNING COUNCIL REPRESENTATIVE FOR AFRICA 13 5.2 UPDATES ON CURE - PROF CAROLINE TIEMESSEN, NATIONAL INSTITUTE FOR COMMUNICABLE DISEASES .......................... 13 6. REGIONAL OVERVIEW OF THE HIV EPIDEMIC – UNAIDS, LAWRENCE MASHIMBYE ................................ -

Research Article the Challenges of Student Affairs at Kenyan Public Universities
Journal of Student Affairs in Africa | Volume 1 (1&2) 2013, 33–48 | ISSN 2307-6267 | DOI: 10.14426/jsaa.v1i1-2.34 research article The challenges of student affairs at Kenyan public universities Tamara Yakaboski* and Matthew Birnbaum** Abstract Kenya is increasingly turning to the promise of mass higher education to help solve a range of economic and social issues. These efforts have had profound effects on university students, faculty and professionals who provide the vital student support services necessary for academic success. This case study explores the challenges that face Kenyan student services professionals within the context of the country’s history and cultures. Kenya’s student service professionals face four major challenges: the increasing costs of attendance, the resulting impact on student behaviours and actions, lack of training and senior leadership, and regular campus closures. Keywords student affairs, accommodation, student housing, student services, university environment, higher education. The challenges of student affairs at Kenyan public universities Kenya is increasingly turning to the promise of mass higher education, meaning a shift from an elite to an open system of access, to help solve a range of economic and social problems (Jowi, 2009; Kenya Vision 2030, 2007). The national government has made its commitment to post-secondary education evident through the addition of over 25 public universities and constituent colleges since 1994 and its adoption of policies encouraging rapid enrolment growth in nearly all post-secondary institutions. Between 2010 and 2013, Kenya made nearly 20 constituent colleges and branch campuses into stand-alone universities. Even with this growing capacity, Kenya’s demand for access to affordable higher education far exceeds the system’s ability to deliver quality instruction and student support (Ngolovoi, 2010; Owuor, 2012). -

Toward an Online Master of Public Health Degree in Kenya: Moi University’S Path
International Journal of Education and Development using Information and Communication Technology (IJEDICT), 2018, Vol. 14, Issue 1, pp. 17-32 Toward an online Master of Public Health degree in Kenya: Moi University’s path Peter Koskei and Rose Ruto-Korir Moi University, Kenya Carol Carrier and Gregory Sales University of Minnesota, USA ABSTRACT As higher education institutions around the world strive to ensure they remain relevant By meeting the needs of today’s digitally-focused students, the numBer of online courses and degree programs is increasing dramatically. Not surprisingly, given this trend, in recent years the School of Public Health at Moi University, in Eldoret, Kenya, has received inquiries from students across Kenya and the neighboring countries of Rwanda, Zambia, and Malawi about the availability of online courses. Recognizing the Benefits to Both potential students and the institution, SPH set a goal to join the trend to offer online courses. This article details SPH’s path toward that end. It discusses the rationale for this decision, examines the state of ICT in Africa in general and Kenya more specifically, the activities undertaken by SPH, resources that have Been devoted to the effort, progress made, challenges faced, and the status of the work to date. Keywords: Online Learning; Online MPH; Kenya; One Health; e-learning INTRODUCTION Officially inaugurated on DecemBer 6, 1985, Moi University has its main campuses in Eldoret, Kenya, was. Growing from a school of agriculture a little over 30 years ago, Moi University now has a total of 15 Schools, 9 Directorates and 2 Institutes. It currently serves more than 52,000 students (Moi University 2017). -

RUFORUM Biennial Conference 2018
The Sixth African Higher Education Week RUFORUM Biennial Conference 2018 22 - 26 October, 2018 | KICC - Kenya Theme: Aligning African Universities to Accelerate Attainment of Africa’s Agenda 2063 Our Motivation “Transforming agriculture in Africa requires innovative scientific research, educational and training approaches. The education sector Our Motivation, further reinforced by the Science needs to be more connected to the new Agenda for Agriculture in Africa challenges facing rural communities and needs to build capacity of young people to be part of the transformation of the agricultural sector” RUFORUM VISION 2030 AT A GLANCE RUFORUM Vibrant, transformative universities catalysing sustainable, inclusive VISION 2030 agricultural development to feed and create prosperity for Africa TAGDev RANCH CREATE K-Hub RUFORUM Transforming African Regional Anchor Cultivating Knowledge Hub Agricultural Universities Universities Research for University Flagship to meaningfully for Agricultural and Teaching Networking, Programmes contribute to Higher Excellence Partnerships and Africa’s Growth and Education Advocacy Development • Student learning: Providing opportunities for transformative student learning. • Research excellence: Creating and advancing knowledge to improve the quality of life. • Community engagement: Serving and engaging society to enhance economic, social and cultural well-being. • Enhancing innovation: Creating opportunities that promote cooperative action among the public, private and civil sectors to leverage resources to RUFORUM stimulate innovation. Commitments • Knowledge generation and sharing: Enhancing knowledge exchange to drive positive change in Africa’s food and agriculture and higher agricultural education systems. • Support to policy dialogue and reform: Connecting and challenging leaders to champion policy innovation, elevate policies as national priorities and catalyse action for transformation. • Fulfilling the potential of women in agricultural science, technology and innovation. -

Unai Members List August 2021
UNAI MEMBER LIST Updated 27 August 2021 COUNTRY NAME OF SCHOOL REGION Afghanistan Kateb University Asia and the Pacific Afghanistan Spinghar University Asia and the Pacific Albania Academy of Arts Europe and CIS Albania Epoka University Europe and CIS Albania Polytechnic University of Tirana Europe and CIS Algeria Centre Universitaire d'El Tarf Arab States Algeria Université 8 Mai 1945 Guelma Arab States Algeria Université Ferhat Abbas Arab States Algeria University of Mohamed Boudiaf M’Sila Arab States Antigua and Barbuda American University of Antigua College of Medicine Americas Argentina Facultad de Ciencias Económicas de la Universidad de Buenos Aires Americas Argentina Facultad Regional Buenos Aires Americas Argentina Universidad Abierta Interamericana Americas Argentina Universidad Argentina de la Empresa Americas Argentina Universidad Católica de Salta Americas Argentina Universidad de Congreso Americas Argentina Universidad de La Punta Americas Argentina Universidad del CEMA Americas Argentina Universidad del Salvador Americas Argentina Universidad Nacional de Avellaneda Americas Argentina Universidad Nacional de Cordoba Americas Argentina Universidad Nacional de Cuyo Americas Argentina Universidad Nacional de Jujuy Americas Argentina Universidad Nacional de la Pampa Americas Argentina Universidad Nacional de Mar del Plata Americas Argentina Universidad Nacional de Quilmes Americas Argentina Universidad Nacional de Rosario Americas Argentina Universidad Nacional de Santiago del Estero Americas Argentina Universidad Nacional de