I PHYTOCHEMICALS and BIOACTIVITIES of PIPER
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dr. Duke's Phytochemical and Ethnobotanical Databases Ehtnobotanical Plants for Gastralgia
Dr. Duke's Phytochemical and Ethnobotanical Databases Ehtnobotanical Plants for Gastralgia Ehnobotanical Plant Common Names Acanthopanax spinosum Wu Chia P'I; Wu Chia; Wu Chia P'I Chiu Alpinia officinarum Kao Liang Chiang; Man Chiang; Kucuk Galanga; Galangal; Liang Chiang Aquilaria agallocha Huang Shu Hsiang; Aguru; Ma T'I Hsiang; Lignum Aloes; Chan Hsiang; Chen Xiang; Ch'En Hsiang; Chi Ku Hsiang; Ch'Ing Kuei Hsiang Brassica integrifolia Brickellia veronicaefolia Canscora diffusa Cedrela mexicana Correa alba Corydalis ambigua Hsuan Hu So; Ezo-Engosaku; Yen Hu So Cyperus rotundus Muskezamin; Tage-tage; Souchet; Hsiang Fu; Woeta; Roekoet teki; Mustaka; Topalak; Musta; So Ts'Ao; So Ken Chiu; Mota; Hsiang Fu Tzu; Boeai; Teki; Mutha; Hama-Suge Dryobalanops aromatica Camphora; Ch'Ing Ping P'Ien; Mei Hua P'Ien; Pokok kapur burus (Baru campho; Ping P'Ien; Ts'Ang Lung Nao; Dryobalanops; Ni Ping P'Ien; Kapur; Su Nao; Chin Chiao Nao; Bing Pian; P'O Lu Hsiang; Mi Nao; Kapurun Elettaria cardamomum Tou K'Ou; Kakule; Ts'Ao Kuo; Krako; Cardamom; Pai Tou K'Ou Euphorbia tirucalli Tikel balung; Milkbush; Pencil Tree; Paching tawa; Tulang-tulang; Mentulang; Kayu urip; Kayu patah tulang (Frac. wood) Foeniculum vulgare Karasu-Uikyo; Shamar; Hui Hsiang Chiu; Finocchio Forte; Tzu Mo; Tzu Mu Lo; Shih Lo; Hsiao Hui Hsiang; Comino; Anis Vert; Rezene; Adas landi; L'Anis; Uikyo; Adas londa; Adas pedas; Anis; La Nuit; Ama-Uikyo; Kaneer Razbana; Fennel; Hinojo; Raziyane; Shbint Hyoscyamus niger Banj Barry; Giusguiamo; Hiyosu; Sukran; Jusquiame; Altercum; Henbane,Black; -

Volatiles of Black Pepper Fruits (Piper Nigrum L.)
molecules Article Volatiles of Black Pepper Fruits (Piper nigrum L.) Noura S. Dosoky 1 , Prabodh Satyal 1, Luccas M. Barata 2 , Joyce Kelly R. da Silva 2 and William N. Setzer 1,3,* 1 Aromatic Plant Research Center, Suite 100, Lehi, UT 84043, USA; [email protected] (N.S.D.); [email protected] (P.S.) 2 Programa de Pós-Graduação em Biotecnologia, Universidade Federal do Pará, Belém 66075-110, PA, Brazil; [email protected] (L.M.B.); [email protected] (J.K.R.d.S.) 3 Department of Chemistry, University of Alabama in Huntsville, Huntsville, AL 35899, USA * Correspondence: [email protected]; Tel.: +1-256-824-6519 Academic Editor: Francesca Mancianti Received: 4 October 2019; Accepted: 5 November 2019; Published: 21 November 2019 Abstract: Black pepper (Piper nigrum) is historically one of the most important spices and herbal medicines, and is now cultivated in tropical regions worldwide. The essential oil of black pepper fruits has shown a myriad of biological activities and is a commercially important commodity. In this work, five black pepper essential oils from eastern coastal region of Madagascar and six black pepper essential oils from the Amazon region of Brazil were obtained by hydrodistillation and analyzed by gas chromatography-mass spectrometry. The major components of the essential oils were α-pinene, sabinene, β-pinene, δ-3-carene, limonene, and β-caryophyllene. A comparison of the Madagascar and Brazilian essential oils with black pepper essential oils from various geographical regions reported in the literature was carried out. A hierarchical cluster analysis using the data obtained in this study and those reported in the literature revealed four clearly defined clusters based on the relative concentrations of the major components. -

Edible Leafy Plants from Mexico As Sources of Antioxidant Compounds, and Their Nutritional, Nutraceutical and Antimicrobial Potential: a Review
antioxidants Review Edible Leafy Plants from Mexico as Sources of Antioxidant Compounds, and Their Nutritional, Nutraceutical and Antimicrobial Potential: A Review Lourdes Mateos-Maces 1, José Luis Chávez-Servia 2,* , Araceli Minerva Vera-Guzmán 2 , Elia Nora Aquino-Bolaños 3 , Jimena E. Alba-Jiménez 4 and Bethsabe Belem Villagómez-González 2 1 Recursos Genéticos y Productividad-Genética, Colegio de Posgraduados, Carr. México-Texcoco Km. 36.5, Montecillo, Texcoco 56230, Mexico; [email protected] 2 CIIDIR-Oaxaca, Instituto Politécnico Nacional, Ciudad de México 07738, Mexico; [email protected] (A.M.V.-G.); [email protected] (B.B.V.-G.) 3 Centro de Investigación y Desarrollo de Alimentos, Universidad Veracruzana, Xalapa-Enríquez 1090, Mexico; [email protected] 4 CONACyT-Centro de Investigación y Desarrollo de Alimentos, Universidad Veracruzana, Xalapa-Enríquez 1090, Mexico; [email protected] * Correspondence: [email protected] Received: 15 May 2020; Accepted: 13 June 2020; Published: 20 June 2020 Abstract: A review of indigenous Mexican plants with edible stems and leaves and their nutritional and nutraceutical potential was conducted, complemented by the authors’ experiences. In Mexico, more than 250 species with edible stems, leaves, vines and flowers, known as “quelites,” are collected or are cultivated and consumed. The assessment of the quelite composition depends on the chemical characteristics of the compounds being evaluated; the protein quality is a direct function of the amino acid content, which is evaluated by high-performance liquid chromatography (HPLC), and the contribution of minerals is evaluated by atomic absorption spectrometry, inductively coupled plasma-optical emission spectrometry (ICP-OES) or ICP mass spectrometry. The total contents of phenols, flavonoids, carotenoids, saponins and other general compounds have been analyzed using UV-vis spectrophotometry and by HPLC. -

Trees and Plants for Bees and Beekeepers in the Upper Mara Basin
Trees and plants for bees and beekeepers in the Upper Mara Basin Guide to useful melliferous trees and crops for beekeepers December 2017 Contents Who is this guide for? .......................................................................................................................................................................................................................................................................... 1 Introduction to the MaMaSe Project .................................................................................................................................................................................................................................................. 1 Market driven forest conservation initiatives in the Upper Mara basin ............................................................................................................................................................................................. 2 Water, apiculture, forests, trees and livelihoods ................................................................................................................................................................................................................................ 3 Types of bees ....................................................................................................................................................................................................................................................................................... 4 How this -
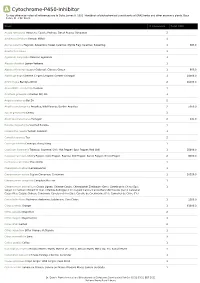
Show Activity
A Cytochrome-P450-Inhibitor *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Acacia farnesiana Huisache; Cassie; Popinac; Sweet Acacia; Opopanax 2 Achillea millefolium Yarrow; Milfoil 1 Acorus calamus Flagroot; Sweetroot; Sweet Calamus; Myrtle Flag; Calamus; Sweetflag 1 384.0 Agastache rugosa 1 Ageratum conyzoides Mexican ageratum 1 Aloysia citrodora Lemon Verbena 1 Alpinia officinarum Lesser Galangal; Chinese Ginger 1 800.0 Alpinia galanga Siamese Ginger; Languas; Greater Galangal 1 24000.0 Ammi majus Bishop's Weed 2 16000.0 Anacardium occidentale Cashew 1 Anethum graveolens Garden Dill; Dill 1 Angelica dahurica Bai Zhi 2 Angelica archangelica Angelica; Wild Parsnip; Garden Angelica 2 5050.0 Apium graveolens Celery 3 Artemisia dracunculus Tarragon 2 141.0 Boronia megastigma Scented Boronia 1 Calamintha nepeta Turkish Calamint 1 Camellia sinensis Tea 2 Cananga odorata Cananga; Ylang-Ylang 1 Capsicum frutescens Tabasco; Cayenne; Chili; Hot Pepper; Spur Pepper; Red Chili 1 35800.0 Capsicum annuum Cherry Pepper; Cone Pepper; Paprika; Bell Pepper; Sweet Pepper; Green Pepper 2 8000.0 Centaurea calcitrapa Star-Thistle 1 Chenopodium album Lambsquarter 1 Cinnamomum verum Ceylon Cinnamon; Cinnamon 1 20320.0 Cinnamomum camphora Camphor; Ho Leaf 1 Cinnamomum aromaticum Cassia Lignea; Chinese Cassia; Chinesischer Zimtbaum (Ger.); Canela de la China (Sp.); 1 Saigon Cinnamon; Chinazimt (Ger.); Kashia-Keihi -
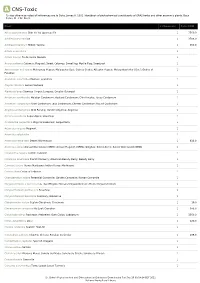
Show Activity
A CNS-Toxic *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Abies sachalinensis Shin-Yo-Yu; Japanese Fir 1 7560.0 Achillea moschata Iva 1 3708.0 Achillea millefolium Milfoil; Yarrow 1 550.0 Acinos suaveolens 1 Acinos alpinus Te de Sierra Nevada 1 Acorus calamus Calamus; Flagroot; Sweet Calamus; Sweetflag; Myrtle Flag; Sweetroot 1 Aframomum melegueta Melegueta Pepper; Malagueta (Sp.); Guinea Grains; Alligator Pepper; Malagettapfeffer (Ger.); Grains-of- 1 Paradise Ageratum conyzoides Mexican ageratum 1 Aloysia citrodora Lemon Verbena 1 Alpinia galanga Siamese Ginger; Languas; Greater Galangal 1 Amomum xanthioides Malabar Cardamom; Bastard Cardamom; Chin Kousha; Tavoy Cardamom 1 Amomum compactum Siam Cardamom; Java Cardamom; Chester Cardamom; Round Cardamom 1 Angelica archangelica Wild Parsnip; Garden Angelica; Angelica 1 Annona squamosa Sugar-Apple; Sweetsop 1 Aristolochia serpentaria Virginia Snakeroot; Serpentaria 1 Artemisia vulgaris Mugwort 1 Artemisia salsoloides 1 Artemisia herba-alba Desert Wormwood 1 638.0 Artemisia annua Annual Wormwood (GRIN); Annual Mugwort (GRIN); Qinghao; Sweet Annie; Sweet Wormwood (GRIN) 1 Calamintha nepeta Turkish Calamint 1 Callicarpa americana French Mulberry; American Beauty Berry; Beauty Berry 1 Cannabis sativa Hemp; Marijuana; Indian Hemp; Marihuana 1 Cedrus libani Cedar of Lebanon 1 Chamaemelum nobile Perennial Camomile; Garden Camomile; Roman Camomile -

Spice Large.Pdf
Gernot Katzer’s Spice List (http://gernot-katzers-spice-pages.com/engl/) 1/70 (November 2015) Important notice Copyright issues This document is a byproduct of my WWW spice pages. It lists names of spices in about 100 different languages as well as the sci- This document, whether printed or in machine-readable form, may entific names used by botanists and pharmacists, and gives for each be copied and distributed without charge, provided the above no- local name the language where it is taken from and the botanical tice and my address are retained. If the file content (not the layout) name. This index does not tell you whether the plant in question is is modified, this should be indicated in the header. discussed extensively or is just treated as a side-note in the context of another spice article. Employees of Microsoft Corporation are excluded from the Another point to make perfectly clear is that although I give my above paragraph. On all employees of Microsoft Corporation, a best to present only reliable information here, I can take no warrant licence charge of US$ 50 per copy for copying or distributing this of any kind that this file, or the list as printed, or my whole WEB file in all possible forms is levied. Failure to pay this licence charge pages or anything else of my spice collection are correct, harm- is liable to juristical prosecution; please contact me personally for less, acceptable for non-adults or suitable for any specific purpose. details and mode of paying. All other usage restrictions and dis- Remember: Anything free comes without guarantee! claimers decribed here apply unchanged. -

“Pimienta” De Los Géneros Piper, Pimenta, Lindera, Ruta, Schin
NOTAS BREVES Botanica Complutensis ISSN-e: 1988-2874 https://dx.doi.org/10.5209/bocm.73020 Composición de aceites esenciales de diferentes especies de “pimienta” de los géneros Piper, Pimenta, Lindera, Ruta, Schinus y Zanthoxylum Héctor Alonso-Miguel1, María José Pérez-Alonso1, Ana Cristina Soria2, Manuel Blanco Martínez1 Resumen. Se ha extraído mediante hidrodestilación el aceite esencial de diez especies usadas como pimienta: Piper borbonense, P. capense, P. retrofractum, P. nigrum, Zanthoxylum bungeanum y Z. armatum, Lindera neesiana, Ruta chalepensis, Schinus terebenthifolia, Pimenta dioica. Los análisis realizados mediante cromatografía de gases acoplada a espectrometría de masas encontraron que todas presentan β-felandreno y derivados de cariofileno y felandreno, siendo estos compuestos de propiedades pungentes los característicos de la especia pimienta. El rendimiento de esencia varía desde 0,43% para R. chalepensis hasta 7,61% para P. borbonense. Los compuestos mayoritarios fueron: P. borbonense (α-felandreno, 12,43%), P. capense (δ-cadineno, 25,59%,), P. retrofractum (γ-cadineno, 31,63%), P. nigrum ((E)-β-cariofileno, 22,88%), P. dioica (eugenol, 48,93%), L. neesiana (miristicina, 14,13%), R. chalepensis (2-undecanona, 64,93%), S. therebenthifolia (δ-3-careno, 29,21%), Z. armatum (linalool, 53,30%); Z. bungeanum (linalool, 64,09%). Todo esto muestra las diferencias en el metabolismo secundario de las pimientas y por tanto sus posibles aplicaciones en diferentes industrias. Palabras clave: Pimienta; especia; aceites esenciales; Piper; Ruta; Pimenta; Lindera; Schinus; Zanthoxylum [en] Composition of essential oils of different species of “pepper” of the Piper, Pimenta, Lindera, Ruta, Schinus and Zanthoxylum genera Abstract.The essential oil of ten species used as pepper has been extracted by hydrodistillation: Piper borbonense, P. -

Periodic Table of Herbs 'N Spices
Periodic Table of Herbs 'N Spices 11HH 1 H 2 HeHe Element Proton Element Symbol Number Chaste Tree Chile (Vitex agnus-castus) (Capsicum frutescens et al.) Hemptree, Agnus Cayenne pepper, Chili castus, Abraham's balm 118Uuo Red pepper 33LiLi 44 Be 5 B B 66 C 7 N 7N 88O O 99 F 1010 Ne Ne Picture Bear’s Garlic Boldo leaves Ceylon Cinnamon Oregano Lime (Allium ursinum) (Peumus boldus) (Cinnamomum zeylanicum) Nutmeg Origanum vulgare Fenugreek Lemon (Citrus aurantifolia) Ramson, Wild garlic Boldina, Baldina Sri Lanka cinnamon (Myristica fragrans) Oregan, Wild marjoram (Trigonella foenum-graecum) (Citrus limon) 11 Na Na 1212 Mg Mg 1313 Al Al 1414 Si Si 1515 P P 16 S S 1717 Cl Cl 1818 Ar Ar Common Name Scientific Name Nasturtium Alternate name(s) Allspice Sichuan Pepper et al. Grains of Paradise (Tropaeolum majus) (Pimenta dioica) (Zanthoxylum spp.) Perilla (Aframomum melegueta) Common nasturtium, Jamaica pepper, Myrtle Anise pepper, Chinese (Perilla frutescens) Guinea grains, Garden nasturtium, Mugwort pepper, Pimento, pepper, Japanese Beefsteak plant, Chinese Savory Cloves Melegueta pepper, Indian cress, Nasturtium (Artemisia vulgaris) Newspice pepper, et al. Basil, Wild sesame (Satureja hortensis) (Syzygium aromaticum) Alligator pepper 1919 K K 20 Ca Ca 2121 Sc Sc 2222 Ti Ti 23 V V 24 Cr Cr 2525 Mn Mn 2626 Fe Fe 2727 Co Co 2828 Ni Ni 29 Cu Cu 3030 Zn Zn 31 Ga Ga 3232 Ge Ge 3333As As 34 Se Se 3535 Br Br 36 Kr Kr Cassia Paprika Caraway (Cinnamomum cassia) Asafetida Coriander Nigella Cumin Gale Borage Kaffir Lime (Capsicum annuum) (Carum carvi) -

Insecticidal Activity of Thai Botanical Extracts Against Development Stages of German Cockroach, Blattella Germanica (L.) (Orthoptera: Blattellidae)
SOUTHEAST ASIAN J TROP MED PUBLIC HEALTH INSECTICIDAL ACTIVITY OF THAI BOTANICAL EXTRACTS AGAINST DEVELOPMENT STAGES OF GERMAN COCKROACH, BLATTELLA GERMANICA (L.) (ORTHOPTERA: BLATTELLIDAE) Soraya Saenmanot1, Ammorn Insung2, Jarongsak Pumnuan2, Apiwat Tawatsin3 Usavadee Thavara3, Atchara Phumee4, Fréderick Gay5, Wanpen Tachaboonyakiat6 and Padet Siriyasatien7 1Medical Science Program, Faculty of Medicine, Chulalongkorn University, Bangkok; 2Department of Pest Management Technology, Faculty of Agricultural Technology, King Mongkut’s Institute of Technology Lat Krabang, Bangkok; 3National Institute of Health, Department of Medical Sciences, Ministry of Public Health, Nonthaburi; 4Thai Red Cross Emerging Infectious Health Science Centre, King Chulalongkorn Memorial Hospital, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand; 5Université Pierre et Marie Curie-Paris 6, CHU Pitié-Salpêtrière, AP-HP, Groupe Hospitalier Pitié-Salpêtrière, Service Parasitologie-Mycologie, Paris, France; 6Department of Materials Science, Faculty of Science, 7Department of Parasitology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand Abstract. The German cockroach, Blattella germanica, is considered an important medical and economic pest in Thailand. The insecticidal activities of hexane, acetone and ethanol extracts derived from six Thai botanicals, namely, Piper ret- rofractum Vahl, Stemona tuberosa Lour, Derris elliptica (Wall.) Benth., Rhinacanthus nasutus (L.), Butea superba Roxb., and Foeniculum vulgare Mill. were used to evaluate -

Report to the Government of Samoa on Invasive Plant Species of Environmental Concern
Report to the Government of Samoa on Invasive Plant Species of Environmental Concern James C. Space and Tim Flynn U.S.D.A. Forest Service Pacific Southwest Research Station Institute of Pacific Islands Forestry Honolulu, Hawai‘i, USA 26 November 2002 Table of Contents Report to the Government of Samoa on Invasive Plant Species of Environmental Concern................................................1 1. Dangerous species not known to be in Samoa..................................................................................................................2 2. Species that are invasive or have the potential to become so in Samoa..........................................................................5 Invasive species already widespread in Samoa ................................................................................................................5 Invasive species of limited extent......................................................................................................................................8 3. Species that are known or listed as weedy or invasive elsewhere and are common, weedy or cultivated in Samoa ....................................................................................................................................................................................11 4. Native species (or Polynesian introductions) exhibiting aggressive behavior..............................................................13 Strategies for dealing with invasive species........................................................................................................................13 -

ROOT BEER PLANT Or MEXICAN PEPPERLEAF Piper Auritum
ROOT BEER PLANT or MEXICAN PEPPERLEAF Piper auritum Characteristics Perennial Spread: 2’ – 12’ Zone: 8 to 12 Leaves: Large, Fragrant, heart-shaped Sun: Partial or dappled shade Large bush or small tree Height: 2’ – 12’ Culture A root beer plant growing in the garden provides an interesting fragrance. A root beer plant, also known as Hoja Santa, holy leaf or Mexican pepperleaf, growing in the garden provides the aroma of root beer, and large, furry leaves in which to wrap foods and give them a hint of root beer flavor. An evergreen shrub or small tree in USDA zones 10 and 11, root beer plants are herbaceous perennials in USDA zones 8 and 9. Flowers of the root beer plant are not showy and sometimes not even noticeable. Plant it in full sun to part shade, feed and water occasionally. Caring for root beer plants can be neglected without the loss of the plant, but the most attractive foliage results from proper care. The plant won’t survive in freezing temperatures. Noteworthy Characteristics Root beer plants are primarily used as culinary ingredients, or in some areas, medicinal. Native to Mexico, this plant has a diversity of uses. Leaves of the root beer plant are steamed and used as wraps in many native dishes. The leaves may also be chopped for use in cooking or salads. Info about root beer plants says they are also used medicinally as an aid to digestion and to calm colicky babies. Other info says it is used for bronchitis and asthma. However, in the United States, the FDA banned its commercial use as root beer flavoring in the 1960’s, as it contains the oil safrole, which is known to be carcinogenic in animals.